In a significant advancement for renewable energy technologies, a new catalyst has been developed that dramatically improves the efficiency and stability of the oxygen evolution reaction (OER) in acidic media, a critical process for water splitting and hydrogen production. The research unearthed a ternary oxide catalyst--Ru3Zn0.85W0.15Ox (RZW)--designed to address the longstanding challenges of achieving high catalytic activity and durability in acidic conditions.
Details of the research were published in the journal Angewandte Chemie International Edition on January 22, 2025.
OER, a key reaction in water splitting, plays a central role in generating green hydrogen, which holds the promise of a sustainable and carbon-free energy solution. However, conventional catalysts often struggle to maintain both high performance and stability in acidic environments. This new catalyst, RZW, harnesses the unique electron-withdrawing properties of tungsten (W) and the sacrificial behavior of zinc (Zn) to enhance OER performance.
The study reveals that during the initial OER process, zinc dissolves from the catalyst, releasing electrons that are captured by tungsten species. This results in electron accumulation at the ruthenium (Ru) sites, enhancing the catalytic activity. Despite the dissolution of zinc, the catalyst maintains its structural integrity and catalytic efficiency, thanks to the stabilizing role of tungsten, which preferentially occupies bridge sites and preserves the active Ru configurations.
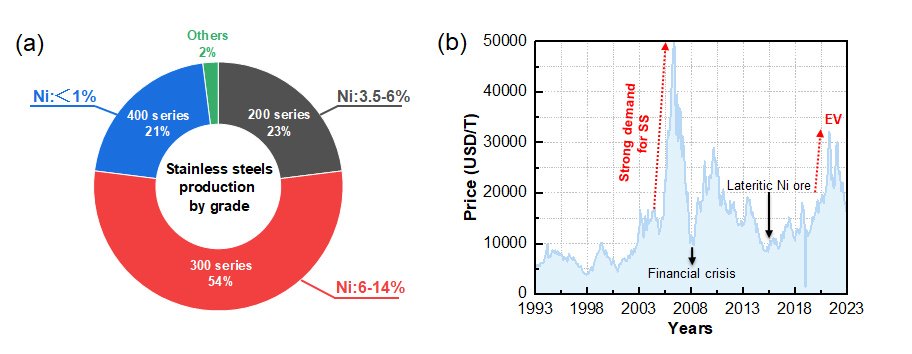
By employing a combination of advanced experimental techniques--including X-ray photoelectron spectroscopy (XPS), high-resolution transmission electron microscopy (HRTEM), and Fourier-transform extended X-ray absorption fine structure (FT-EXAFS)--alongside theoretical density functional theory (DFT) calculations, the research team investigated the structural and electronic properties of the catalyst under OER conditions. The findings show that the rapid dissolution of zinc significantly contributes to enhanced electron transfer, improving both the OER activity and long-term stability of the catalyst.
"This breakthrough demonstrates how strategic doping with tungsten and the use of sacrificial metals like zinc can greatly improve the performance of OER catalysts," said Hao Li, Associate Professor at Tohoku University's Advanced Institute for Materials Research (WPI-AIMR) and corresponding author of the paper. "Our findings suggest that this approach offers a promising pathway for developing high-performance, cost-effective catalysts for green hydrogen production, which is crucial in the transition to renewable energy."
The research has been made available through the Digital Catalysis Platform (DigCat), the largest experimental catalysis database to date, developed by the Hao Li Lab.
The article processing charge (APC) was supported by the Tohoku University Support Program.
The next step for this research is to test the RZW catalyst in full electrolyzer systems to assess its performance in real-world applications. By bridging the gap between fundamental research and practical implementation, the team aims to contribute to the development of more efficient and scalable hydrogen production technologies.
- Publication Details:
Title: W-mediated electron accumulation in Ru-O-W motifs enables ultra-stable oxygen evolution reaction in acid
Authors: Kai Zhou, Heng Liu, Zhongliang Liu, Xiaoning Li, Nana Wang, Mingyue Wang, Tianrui Xue, Yongjun Shen, Hao Li, Huihui Li1, Chunzhong Li.
Journal: Angewandte Chemie International Edition






