Joint press release by Hokkaido University and University of Technology Sydney.
A catalyst that significantly enhances ammonia conversion could improve wastewater treatment, green chemical and hydrogen production.

Scanning electron micrograph of the catalyst, NiOOH-Ni, developed in this study. (Hanwen Liu, et al. Advanced Energy Materials. August 7, 2024)
A team of scientists have developed an effective catalyst with a remarkable ability to enhance the efficiency of ammonia conversion. Published in Advanced Energy Materials, the study reveals the catalyst's potential to significantly advance wastewater treatment, green nitrite and nitrate, as well as hydrogen production.
Catalysts are substances that speed up chemical reactions by providing a more efficient route for a reaction to occur and making it easier to start and finish. Since catalysts are neither consumed nor altered in the reaction, they can be used repeatedly, and they are essential in a variety of industrial, environmental, and biochemical processes.
The team, which included researchers from Japan's Hokkaido University, Australia's University of Technology Sydney and elsewhere, developed the catalyst, called NiOOH-Ni, by combining nickel (Ni) with nickel oxyhydroxide.
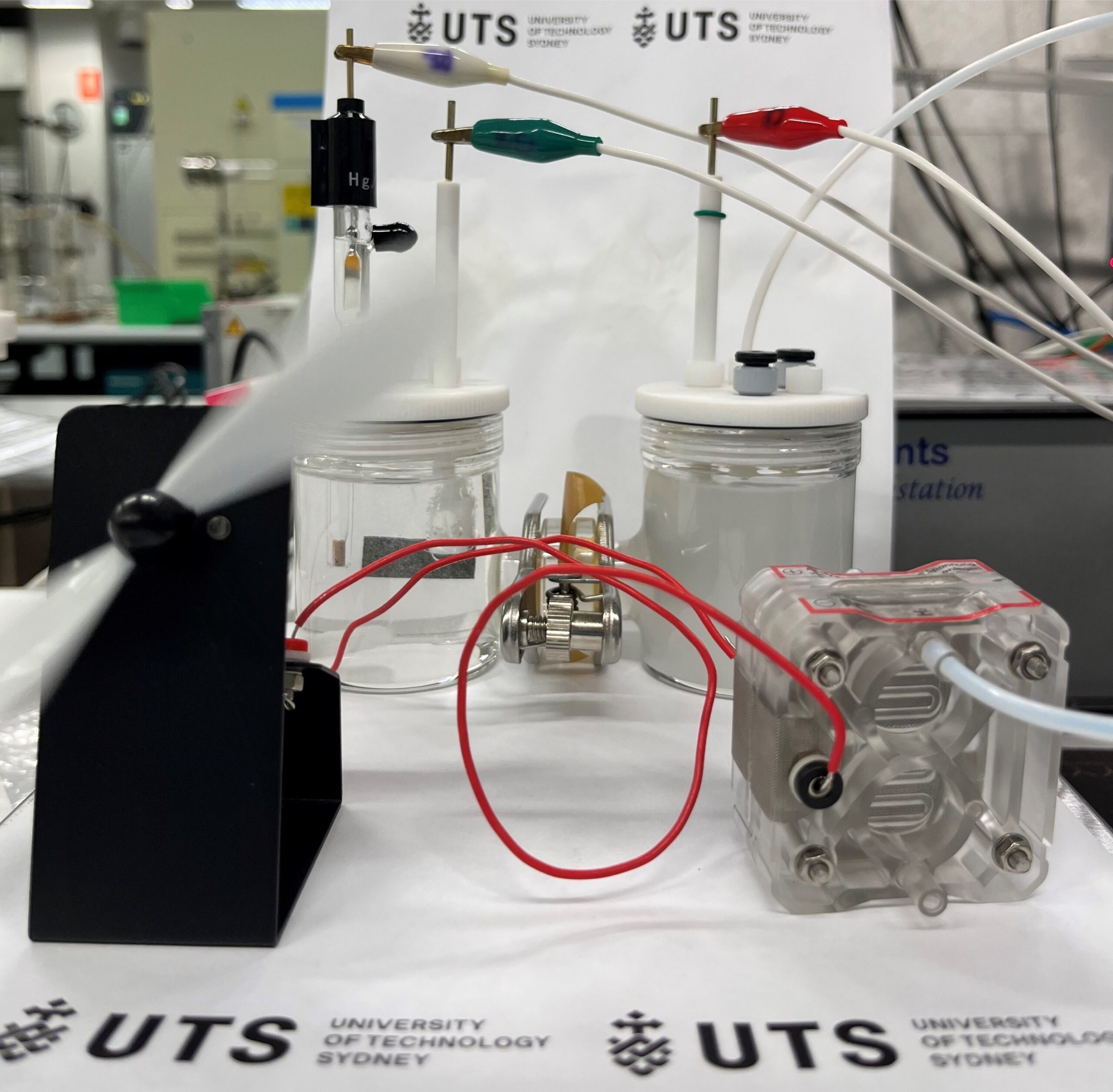
The reaction cell for simultaneous hydrogen production and ammonia oxidation. (Hanwen Liu, et al. Advanced Energy Materials. August 7, 2024)
Ammonia can cause severe environmental problems, such as excessive algal growth in water bodies, which depletes oxygen and harms aquatic life. At high concentrations, ammonia can harm humans and wildlife. Effective management and conversion of ammonia are thus critical, but its corrosive nature makes it difficult to handle.
The researchers developed NiOOH-Ni using an electrochemical process. Nickel foam, a porous material, was treated with an electrical current while immersed in a chemical solution. This treatment resulted in the formation of nickel oxyhydroxide particles on the foam's surface. Despite their irregular and non-crystalline structure, these nickel-oxygen particles significantly enhance ammonia conversion efficiency. The catalyst's design allows it to operate effectively at lower voltages and higher currents than traditional catalysts.
"NiOOH-Ni works better than Ni foam, and the reaction pathway depends on the amount of electricity (voltage) used," explains Professor Zhenguo Huang from the University of Technology Sydney, who led the study. "At lower voltages, NiOOH-Ni produces nitrite, while at higher voltages, it generates nitrate."
This means the catalyst can be used in different ways depending on what is needed. For example, it can be used to clean wastewater by converting ammonia into less harmful substances. But in another process, it can also be used to produce hydrogen gas, a clean fuel. This flexibility makes NiOOH-Ni valuable for various applications.
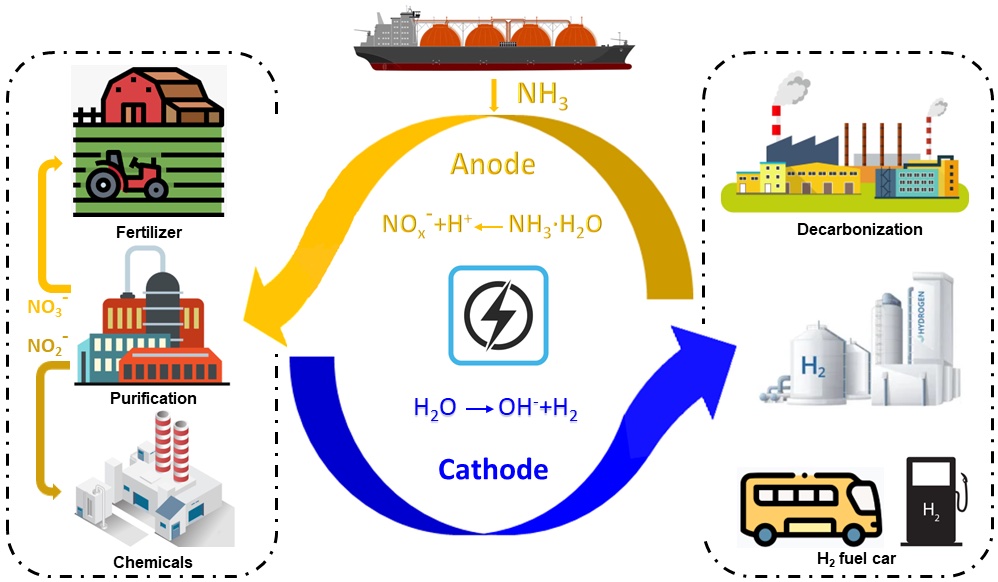
Electrolysis of ammonia aqueous solution produces nitrite and nitrate on the NiOOH-Ni anode, and green hydrogen on the Ni2P-Ni cathode. This presents advantages over the currently used thermal ammonia cracking due to the simultaneous formation of hydrogen as an energy carrier and nitrite and nitrate as valuable chemicals under ambient conditions. (Hanwen Liu, et al. Advanced Energy Materials. August 7, 2024)
"NiOOH-Ni is impressively durable and stable, and it works well even after being used multiple times," says Associate Professor Andrey Lyalin from Hokkaido University, who was involved in the study. "This makes it a great alternative to traditional, more expensive catalysts like platinum, which aren't as effective at converting ammonia."
The catalyst's long-term reliability makes it suitable for large-scale industrial use, potentially transforming how industries handle wastewater and produce clean energy.
Original Article:
Hanwen Liu, et al. Electrocatalytic Ammonia Oxidation to Nitrite and Nitrate with NiOOH-Ni. Advanced Energy Materials. August DD, 2024.
DOI: 10.1002/aenm.202401675
Funding:
This work was supported by the Australian Research Council's Discovery Projects funding scheme (DP220103458) and the Future Fellowship (FT190100658); the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) Program: Data Creation and Utilization-Type Material Research and Development Project (JPMXP1122712807); the Institute for Solid State Physics, the University of Tokyo, Japan; and, the Research Center for Computational Science, Okazaki, Japan (23-IMS-C016).






