With the advantages of abundant resources, low cost and high safety, sodium ion batteries (SIBs) have broad application prospects. NaVPO4F is regarded as one of the most competitive cathodes in high-performance SIBs.
Two polymorphs with the tetragonal and monoclinic NaVPO4F have been reported. However, the accurate crystal structure, the transformation mechanism and the Na-storage dynamic of two-phase NaVPO4F have not been well studied, limiting further application of NaVPO4F in SIBs.
Recently, a research group led by Prof. LI Xianfeng and Prof. ZHENG Qiong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) revealed the irreversible phase transition mechanism under variable temperature and sodium storage kinetics of monoclinic and tetragonal NaVPO4F.
This study was published in Advanced Energy Materials on April 22.
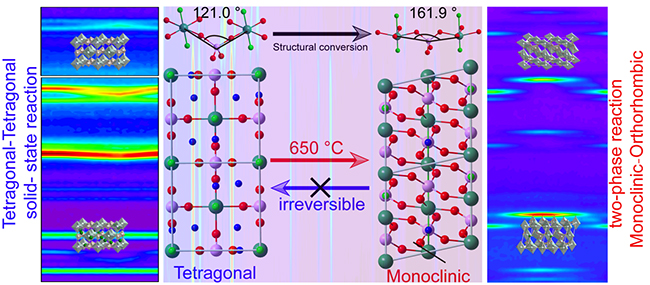
The researchers obtained tetragonal and monoclinic NaVPO4F with high crystallinity and purity by low temperature hydrothermal and high temperature calcination.
"We first observed the accurate cell structure of the two phases in atomic scale, the cell boundary of tetragonal phase is square, while that of monoclinic phase is parallelogram," said Prof LI. "TheNaVPO4F crystal undergoes an irreversible crystal conversion from tetragonal to monoclinic phase."
Moreover, they revealed the difference of binding energy caused by different V-P-V bond angles in tetragonal and monoclinic phases. Compared with tetragonal NaVPO4F, monoclinic phase exhibited better thermal stability due to its higher bond binding energy. As a result, the irreversible phase transition from tetragonal to monoclinic phase would occur when the temperature rises above 650 ℃.
The researchers also analyzed the Na-storage mechanism and electrochemical kinetics of the two crystal structures. They confirmed that the solid solution reaction process of tetragonal phase and "monoclinic to orthorhombic" two-phase transition reaction of monoclinic phase were in the electrochemical reaction, proving better electrochemical stability of tetragonal NaVPO4F.
In terms of kinetics, monoclinic NaVPO4F had higher intrinsic conductivity and sodium ion diffusion rate, showing higher power density, while the intrinsic Na-extraction activation energy of tetragonal NaVPO4F was higher, which was attributed to the higher working voltage and higher energy density.
As a result, monoclinic NaVPO4F could be employed as electrode in priority for high-power-density type SIBs, and tetragonal NaVPO4F could be used as the candidate electrode for high-energy-density type SIBs.
This study provides theoretical basis and technical support for the designing of high-performance sodium ion battery electrodes and the development of next generation sodium ion battery systems with high-energy-density and high-power-density.
The above work was supported by the Strategic Priority Research Program of CAS, the National Natural Science Foundation of China, the DNL Cooperation Fund of CAS, and the Youth Innovation Promotion Association CAS.






