To clarify the early evolutionary history of Cicadoidea fossils, the phylogenetic relationships between Mesozoic fossils and extant Cicadoidea, the macroevolution of body structure adaptations, and their relationship with environmental changes, Dr. JIANG Hui, supervised by Profs. WANG Bo and ZHANG Haichun from the Nanjing Institute of Geology and Palaeontology of the Chinese Academy of Sciences (NIGPAS), together with collaborators, conducted a collaborative cicada study.
The results were published in Nature Communications.
Cicadas, here referring to the superfamily Cicadoidea, are known for their evolution of a sound production system, exceptional long-term subterranean habits, and symbolic attributes and utility in research that widely exist in culture, life, and science. Extant Cicadoidea include the globally distributed Cicadidae, commonly known as true/singing cicadas, and relictual Tettigarctidae, found only in Australia and colloquially known as hairy cicadas.
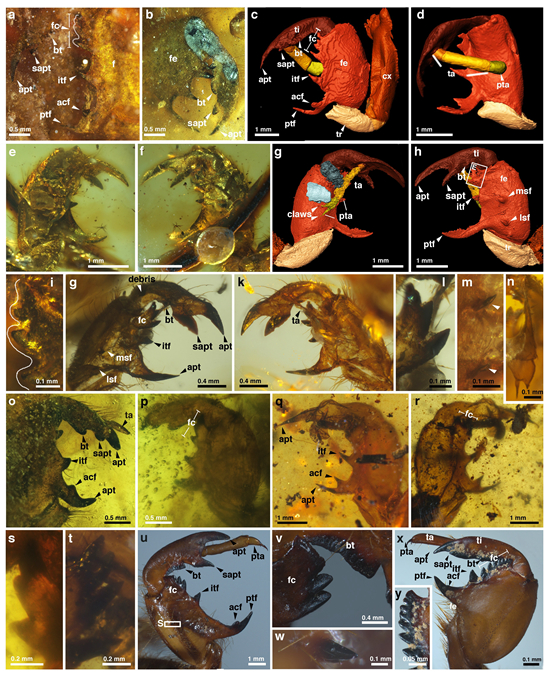
Currently, the earliest fossil of Cicadoidea was found in the Triassic. All Mesozoic (approximately 252 to 66 million years ago) Cicadoidea fossils have traditionally been divided into Cicadidae and Tettigarctidae based on a few distinct and conservative morphological features. However, this direct assignment of Mesozoic fossils to modern taxa may overlook the role of unique and transitional features provided by fossils in tracing their early evolutionary paths.
In this study, the researchers examined the phylogenetic relationships of fossil and extant Cicadoidea groups for the first time. They found that Mesozoic Cicadoidea fossils include stem cicadoids, stem tettigarctids, and stem cicadids. Some Mesozoic fossils previously classified as Tettigarctidae may actually be phylogenetically closer to modern Cicadidae. The Cicadidae and Tettigarctidae clades may have diverged at or by the Middle Jurassic. Due to preservation problems, the classification of insect fossils often relies on the preserved partial morphological features.
They conducted a morphological analysis of the partial structures of adults and nymphs, such as wings, non-wing body parts, and nymphal legs, to compare subtle continuous morphological changes with classification and phylogenetic results, and revealed that specialized homologous structures in insect fossils may contain previously overlooked identifiable transitional variation.
Closer examination of these continuous morphological changes can provide a more precise understanding of the impact of spatiotemporal changes on morphological evolution and further clarify patterns of macroevolution. For example, changes in the head and labium may reflect resistance adaptations due to feeding pressure from changes in host plants. In addition, changes in the thoracic notum, and changes in wing venation and outline, may indicate the evolution of flight muscles and capabilities. Changes in the head and thorax can also be quantified and compared.
Sound production is an important communication method for many animals. Modern Cicadidae species can produce the loudest sounds among insects, reaching nearly 120 decibels through tymbal mechanisms. In contrast, Tettigarctidae communicate with more subtle vibrational signals transmitted through the substrate and lack the ability to produce loud sounds. The different sound-producing mechanisms between Cicadidae and Tettigarctidae ignite curiosity about the initial evolution of their acoustic structures and behaviors.
Tymbals were identified in all Mesozoic cicadoid stem groups, preserved in both male and female specimens. This is the first identification of tymbal structures in Cicadoidea fossils, capturing this communication method in the fossil record. The majority of relatively intact fossils lacked elements for intricate sound production and auditory systems, suggesting mid-Cretaceous cicadoids may have relied on substrate-transmitted vibrations for communication, rather than producing or perceiving high-decibel songs.
In addition, there are cases where the discovery of tymbal muscles and an abdominal cavity in a fossil, along with preserved tracheae, flight muscles, and Marplesian tubes, suggests the possibility of an inherent abdominal cavity and resonant capabilities similar to those found in the abdomens of modern singing cicadas.
Consequently, the researchers proposed another hypothesis: certain mid-Cretaceous Cicadoidea groups may have produced sounds louder than substrate-transmitted vibrations. In any case, compared to modern singing cicadas, Cicadoidea species may have been relatively silent during most of the Mesozoic.
They also reported the oldest known Cicadoidea nymph and exuviae fossils from mid-Cretaceous Kachin amber. The strikingly strong fossorial forelegs of these nymph fossils, similar to those of modern cicadas, suggest similar behaviors and robust capabilities for digging, soil transport, and subterranean living. Cicada nymph and adult fossils show distinct ecological niches and survival strategies, with a notable shift from underground root feeding to aboveground stem feeding.
Evidence of root feeding is rare in fossils. However, fossils of cicada nymphs with specialized digging forelegs suggest this behavior. This subterranean lifestyle presumably provided a survival advantage, allowing cicada nymphs to spend extended periods underground.
The researchers also examined the occurrence of root feeding among arthropods in the fossil record. Given the adult and nymph Cicadoidea fossils from the Kachin amber and the abundance of adult fossils from the Middle Jurassic Daohugou deposit, it's clear that mid-Mesozoic Cicadoidea exhibited distinct life-stage niches, facilitated the transfer of biomass transfer from underground to aboveground, and influenced ecosystems in a manner similar to their modern counterparts.
This work was funded by the National Natural Science Foundation of China and CAS.