Metabolic imaging is a noninvasive method that enables clinicians and scientists to study living cells using laser light, which can help them assess disease progression and treatment responses.
But light scatters when it shines into biological tissue, limiting how deep it can penetrate and hampering the resolution of captured images.
Now, MIT researchers have developed a new technique that more than doubles the usual depth limit of metabolic imaging. Their method also boosts imaging speeds, yielding richer and more detailed images.
This new technique does not require tissue to be preprocessed, such as by cutting it or staining it with dyes. Instead, a specialized laser illuminates deep into the tissue, causing certain intrinsic molecules within the cells and tissues to emit light. This eliminates the need to alter the tissue, providing a more natural and accurate representation of its structure and function.
The researchers achieved this by adaptively customizing the laser light for deep tissues. Using a recently developed fiber shaper - a device they control by bending it - they can tune the color and pulses of light to minimize scattering and maximize the signal as the light travels deeper into the tissue. This allows them to see much further into living tissue and capture clearer images.
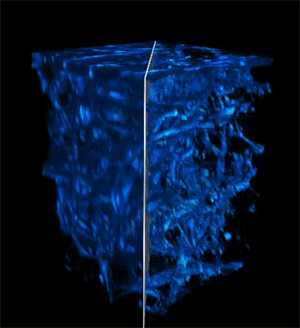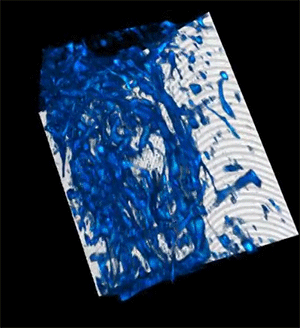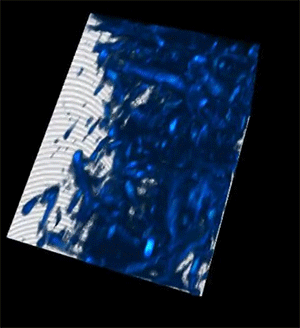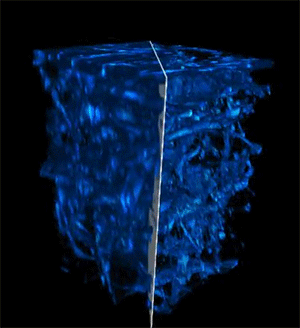
Credit: Courtesy of the researchers
Greater penetration depth, faster speeds, and higher resolution make this method particularly well-suited for demanding imaging applications like cancer research, tissue engineering, drug discovery, and the study of immune responses.
"This work shows a significant improvement in terms of depth penetration for label-free metabolic imaging. It opens new avenues for studying and exploring metabolic dynamics deep in living biosystems," says Sixian You, assistant professor in the Department of Electrical Engineering and Computer Science (EECS), a member of the Research Laboratory for Electronics, and senior author of a paper on this imaging technique.
She is joined on the paper by lead author Kunzan Liu, an EECS graduate student; Tong Qiu, an MIT postdoc; Honghao Cao, an EECS graduate student; Fan Wang, professor of brain and cognitive sciences; Roger Kamm, the Cecil and Ida Green Distinguished Professor of Biological and Mechanical Engineering; Linda Griffith, the School of Engineering Professor of Teaching Innovation in the Department of Biological Engineering; and other MIT colleagues. The research appears today in Science Advances .
Laser-focused
This new method falls in the category of label-free imaging, which means tissue is not stained beforehand. Staining creates contrast that helps a clinical biologist see cell nuclei and proteins better. But staining typically requires the biologist to section and slice the sample, a process that often kills the tissue and makes it impossible to study dynamic processes in living cells.
In label-free imaging techniques, researchers use lasers to illuminate specific molecules within cells, causing them to emit light of different colors that reveal various molecular contents and cellular structures. However, generating the ideal laser light with certain wavelengths and high-quality pulses for deep-tissue imaging has been challenging.
The researchers developed a new approach to overcome this limitation. They use a multimode fiber, a type of optical fiber which can carry a significant amount of power, and couple it with a compact device called a "fiber shaper." This shaper allows them to precisely modulate the light propagation by adaptively changing the shape of the fiber. Bending the fiber changes the color and intensity of the laser.
Building on prior work , the researchers adapted the first version of the fiber shaper for deeper multimodal metabolic imaging.
"We want to channel all this energy into the colors we need with the pulse properties we require. This gives us higher generation efficiency and a clearer image, even deep within tissues," says Cao.
Once they had built the controllable mechanism, they developed an imaging platform to leverage the powerful laser source to generate longer wavelengths of light, which are crucial for deeper penetration into biological tissues.
"We believe this technology has the potential to significantly advance biological research. By making it affordable and accessible to biology labs, we hope to empower scientists with a powerful tool for discovery," Liu says.
Dynamic applications
When the researchers tested their imaging device, the light was able to penetrate more than 700 micrometers into a biological sample, whereas the best prior techniques could only reach about 200 micrometers.
"With this new type of deep imaging, we want to look at biological samples and see something we have never seen before," Liu adds.
The deep imaging technique enabled them to see cells at multiple levels within a living system, which could help researchers study metabolic changes that happen at different depths. In addition, the faster imaging speed allows them to gather more detailed information on how a cell's metabolism affects the speed and direction of its movements.
This new imaging method could offer a boost to the study of organoids, which are engineered cells that can grow to mimic the structure and function of organs. Researchers in the Kamm and Griffith labs pioneer the development of brain and endometrial organoids that can grow like organs for disease and treatment assessment.
However, it has been challenging to precisely observe internal developments without cutting or staining the tissue, which kills the sample.
This new imaging technique allows researchers to noninvasively monitor the metabolic states inside a living organoid while it continues to grow.
With these and other biomedical applications in mind, the researchers plan to aim for even higher-resolution images. At the same time, they are working to create low-noise laser sources, which could enable deeper imaging with less light dosage.
They are also developing algorithms that react to the images to reconstruct the full 3D structures of biological samples in high resolution.
In the long run, they hope to apply this technique in the real world to help biologists monitor drug response in real-time to aid in the development of new medicines.
"By enabling multimodal metabolic imaging that reaches deeper into tissues, we're providing scientists with an unprecedented ability to observe nontransparent biological systems in their natural state. We're excited to collaborate with clinicians, biologists, and bioengineers to push the boundaries of this technology and turn these insights into real-world medical breakthroughs," You says.
"This work is exciting because it uses innovative feedback methods to image cell metabolism deeper in tissues compared to current techniques. These technologies also provide fast imaging speeds, which was used to uncover unique metabolic dynamics of immune cell motility within blood vessels. I expect that these imaging tools will be instrumental for discovering links between cell function and metabolism within dynamic living systems," says Melissa Skala, an investigator at the Morgridge Institute for Research who was not involved with this work.
"Being able to acquire high resolution multi-photon images relying on NAD(P)H autofluorescence contrast faster and deeper into tissues opens the door to the study of a wide range of important problems," adds Irene Georgakoudi, a professor of biomedical engineering at Tufts University who was also not involved with this work. "Imaging living tissues as fast as possible whenever you assess metabolic function is always a huge advantage in terms of ensuring the physiological relevance of the data, sampling a meaningful tissue volume, or monitoring fast changes. For applications in cancer diagnosis or in neuroscience, imaging deeper - and faster - enables us to consider a richer set of problems and interactions that haven't been studied in living tissues before."
This research is funded, in part, by MIT startup funds, a U.S. National Science Foundation CAREER Award, an MIT Irwin Jacobs and Joan Klein Presidential Fellowship, and an MIT Kailath Fellowship.






