A new highly effective thrombosis inhibitor is in sight: Würzburg scientists present promising inhibitor EMA601 for efficient prevention and treatment of arterial thrombosis and inflammatory reactions without increased bleeding risk.
An unhealthy lifestyle, diseases or injuries, genetic predisposition, and increased coagulation tendency can promote the formation of thrombi in blood vessels. These clots obstruct the flow of blood to vital organs, which may lead to life-threatening infarction. Therefore, preventing and treating thrombosis is crucial to avoid severe complications.
Professor Bernhard Nieswandt, Head of the Chair of Experimental Biomedicine I at the University Hospital Würzburg in Germany (UKW) and Research Group Leader at the Rudolf Virchow Center (RVZ) of the University of Würzburg (JMU), has published a study with his team in the European Heart Journal on a new, highly potent, and low-side-effect antithrombotic agent that could be used broadly in therapeutic applications.
Surface Receptor Plays a Key Role in Platelet Activation and Aggregation
Bernhard Nieswandt has been focusing on platelets, since the beginning of his scientific career and has been a pioneer in this field. Twenty-five years ago, he was the first to describe the function of the receptor glycoprotein VI, or GPVI for short, which is found exclusively in platelets and their precursor cells in the bone marrow.
This surface receptor is primarily responsible for the binding of collagen on the injured vessel wall, triggering the activation and aggregation, or clotting, of platelets. This allows the small, nucleus-free cells, only a thousandth of a millimeter in size, with about 250 million in each milliliter of blood, to fulfil their central role in hemostasis and curbing bleeding after injuries.
However, excessive activation of GPVI can lead to the formation of pathological thrombi and thus to vascular occlusions and acute life-threatening events such as heart attacks or strokes. In addition, platelets play a pivotal role in inflammatory reactions that can damage vital organs, with GPVI being of fundamental importance here as well.
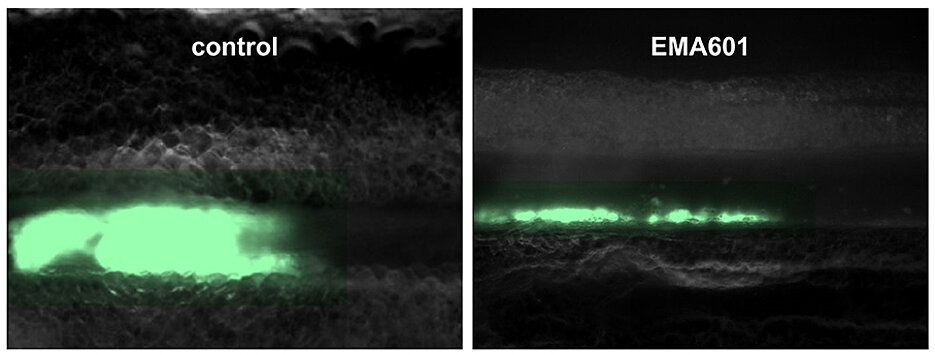
"The Würzburg Platelet Group's research of the past 25 years has impressively shown that GPVI is a promising target for anti-thrombotic and anti-inflammatory therapies, laying the foundation for the development and clinical testing of GPVI inhibitors," explains Bernhard Nieswandt. The biologist discovered a mechanism by which GPVI can be both inhibited and turned off. An initial drug, the GPVI inhibitor ACT017/Glenzocimab, has already been developed up to clinical phase III trials by a French research group based on fundamental research from Würzburg University Medicine: "Recently published first clinical data with this GPVI inhibitor in stroke patients, are very promising and indicate that this therapeutic approach can work in humans," says Bernhard Nieswandt.
His team has also developed a GPVI-blocking antibody that far exceeds the effectiveness of previous agents, even at very low doses, without increasing the risk of bleeding. "Our antibody is 50 times more potent than the previously described GPVI inhibitors and is likely to have higher clinical efficacy and a wider range of applications," Bernhard Nieswandt is convinced.
Late-Breakthrough Abstract at the ISTH Congress in Bangkok
The GPVI inhibitor EMA601 was developed by the Bavarian biotech company EMFRET Analytics, co-founded by Bernhard Nieswandt. EMA601 was functionally investigated by researchers from UKW, RVZ, and JMU, in particular Dr. Stefano Navarro. As part of his doctoral thesis, he analysed antibody fragments from mice as well as humanised antibodies in vitro and in vivo and is the first author of the publication. The scientist presented his findings as a late-breakthrough presentation at the ISTH Congress (Congress of the International Society on Thrombosis and Haemostasis) in Bangkok (Thailand) in June.
Stefano Navarro's conclusion: "EMA601 is a conceptually novel and promising agent for the treatment and secondary prophylaxis of blood clots to prevent infarctions, but also for suppressing inflammatory processes caused by platelets that can damage vital organs."
Bernhard Nieswandt explains the clinical background: "Using the example of a stroke, secondary prophylaxis means that after successful removal of the embolus that has migrated to the brain and disrupted the blood supply, the source of the embolus still needs to be treated antithrombotically. But even if blood flow has been restored to the heart or brain after an infarction, the platelets, as modulators of the immune system, can still drive inflammation and promote tissue damage." The term thrombo-inflammation was coined in Würzburg for these inflammatory processes.
Another significant advantage of the antibody EMA601 is that it does not seem to impair normal blood coagulation, whereas conventional antithrombotic agents currently in clinical use are associated with an increased bleeding tendency. Bernhard Nieswandt: "In neurology, our colleagues still see a considerable number of patients whose heart attack was well-treated cardiologically but who then suddenly experience a brain hemorrhage. For this clinical situation, the GPVI inhibitor could also be a significant advancement."
Publication
Stefano Navarro, Ivan Talucci, Vanessa Göb, Stefanie Hartmann, Sarah Beck, Valerie Orth, Guido Stoll, Hans M Maric, David Stegner, Bernhard Nieswandt, The humanized platelet glycoprotein VI Fab inhibitor EMA601 protects from arterial thrombosis and ischaemic stroke in mice, European Heart Journal, 2024; ehae482, https://doi.org/10.1093/eurheartj/ehae482






