

Aoki (left; corresponding author) and Abe (right; first author) holding plants and commercially available protective glasses made of polycarbonate
On October 28th, Tokyo Institute of Technology held an online press briefing with Assistant Professor Daisuke Aoki of the Department of Chemical Science and Engineering, School of Materials and Chemical Technology. He discussed his work on a method to convert plastic into fertilizer, a development that could contribute to solving major global issues.
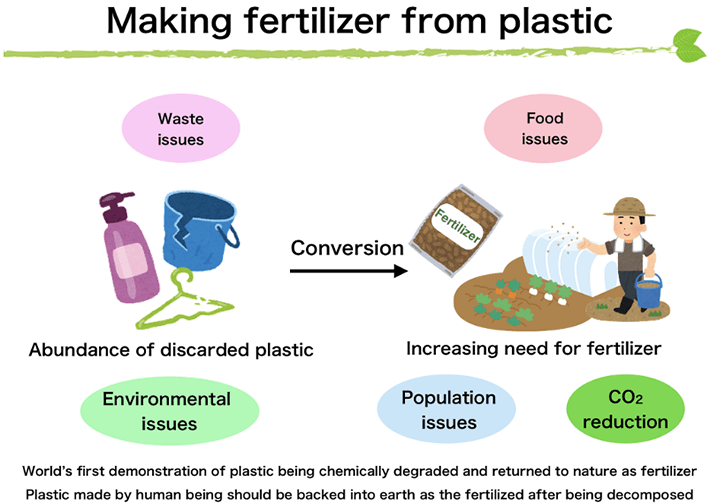
The research concept
Background:
Over 70% of plastic is discarded; only about 15% is recycled
Plastic is found everywhere in daily life. Because plastics are inexpensive and light, they are used to make shopping bags, drink bottles, and even vehicle components. Since plastics deform when heated, they can easily be shaped and produced in bulk. They are made of resins, which are polymers that contain carbon. By changing the chemical structure and topology of the molecular chain, plastics can be made as flexible or as tough as the application requires. For this reason, plastics have helped enrich our lives.
Aoki explaining the uses of plastics and related issues
Research on plastics with new properties continue. But recently, the focus has shifted to the degradation and recyclability of materials in addition to developing new functionality. This is because in general, over 70% of plastic is discarded, and only about 15% is recycled. There is also the problem of small particles of plastic—microplastics—finding their way into the oceans. Aoki pointed out that the demand for plastics is extremely great, and that reducing their disposal and utilizing them effectively are a pressing global issue.
Point 1: Degrading bio-based plastic with aqueous ammonia to produce urea
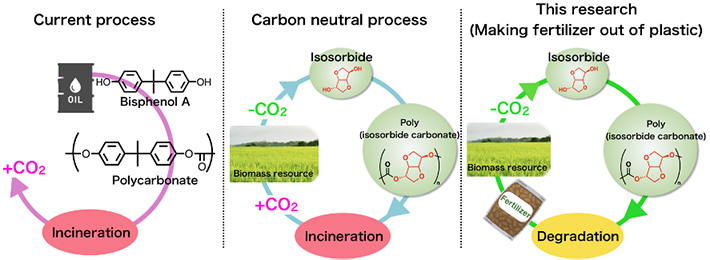
Instead of the conventional practice (in Japan) of incinerating discarded plastic, Aoki and colleagues developed a degradation method that minimizes carbon dioxide emissions and proposed a system to recycle the plastic. The system takes bio-based plastic and degrades it with aqueous ammonia, resulting in a product that can be used as fertilizer. Degradation with aqueous ammonia does not require carbon-emitting energy or catalysts made of expensive precious metals. Further, the product can be used as fertilizer without further processing, achieving an ideal eco-friendly cycle.
A condition for this system is that the starting material for the plastic must be safe and non-toxic: the plastic must not be the conventional petroleum-based type, but come from an organic source—biomass. Specifically, the system uses plastic called "poly(isosorbide carbonate)." Aoki related that this was a concept he had nurtured since he applied to the Techno-Renaissance Japan idea contest as a student and that he was able to publish it as an academic paper now, 10 years later, after succeeding in this demonstration.
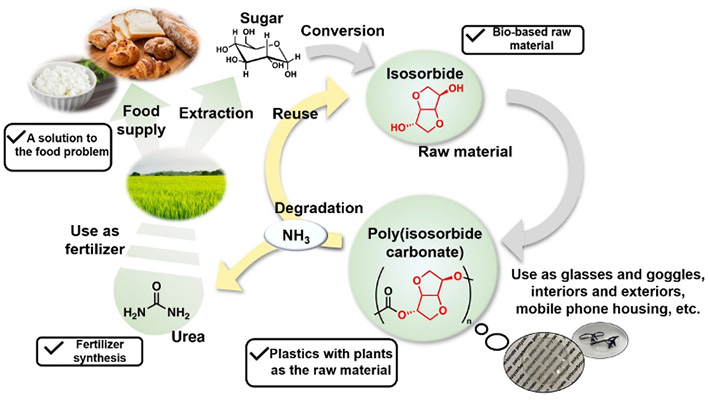
Overview of the recycling system for bio-based plastic
A polycarbonate (a polymer that is linked by carbonate groups) is a type of plastic, and it can be completely degraded by ammonia so that the polymer (multiple monomers connected by chemical bonds) can be converted into monomers and urea. Therefore, if safe monomers are used to make the polycarbonate, it will degrade into a mixture of safe monomers and urea. The team therefore used isosorbide (a non-toxic compound synthesized from glucose), which is a resource from biomass, as the monomer to make poly(isosorbide carbonate), a polymer that is isosorbide linked by carbonate groups.
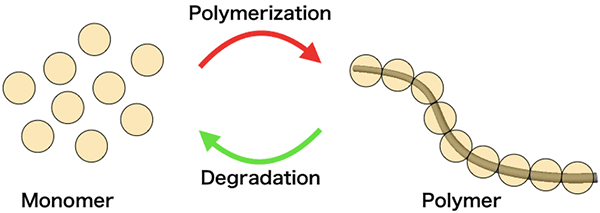
Monomers and polymer
To demonstrate the recycling system, the team reacted the synthesized carbonate with aqueous ammonia and studied how it degraded. The cloudy plastic dispersion liquid became a clear liquid. This indicates that the monomers linked by carbonate groups were separated by the aqueous ammonia. In other words, degradation had occurred, converting the plastic into monomers and urea. Aoki explained that, when the team optimized the reaction conditions for the degradation of carbonates, they found that degradation using aqueous ammonia heated to 90°C was the most effective.

Changes a polycarbonate undergoes as it is degraded by aqueous ammonia
Point 2: Demonstrating that the urea and isosorbide product function as fertilizer
Urea is one of the world's most important chemical compounds, said to be sustaining half of the global population through its use as fertilizer. The research team used the degraded components, including urea, and experimented with their effect on plant growth. They investigated the growth of thale cress (Arabidopsis thaliana) under three conditions—isosorbide only, urea only, and the whole degradation product—and found that the thale cress given the whole degradation product grew the most.

Test on the growth of thale cress (Arabidopsis thaliana)
Aoki also described the growth of mitsuba (Cryptotaenia japonica), a plant familiar as a vegetable, which was a preliminary result for consideration that was not included in the paper.
He concluded: "The Haber-Bosch process produces ammonia, an ingredient of fertilizer, by reacting the nitrogen and hydrogen in the air directly. It was heralded as a radical invention, described as 'making bread from air.' We want to further our research to create a process to 'make bread from plastic.'"

Testing on the growth of mitsuba; the team will study the growth mechanisms and types of plants for which their system is suited
Comment by Assistant Professor Daisuke Aoki and Takumi Abe (1st-year master's student, School of Materials and Chemical Technology)
The recycling system we demonstrated in this study can be applied to bio-based polycarbonates. The volume of this type of plastic among the plastics widely available in the market today is limited. But this concept of taking bio-based polycarbonate and chemically recycling it into fertilizer through degradation instead of incinerating it allows us to envision a next-generation recycling system that goes beyond carbon neutrality and uses up plastic as it reduces carbon dioxide. We expect that it will contribute to achieving a "decarbonized society," making progress in addressing plastic and food issues, and achieving SDGs. Our wish is to implement this recycling system in society through industry-government-academia collaboration. Taking plastic and making fertilizer, growing wheat, then making bread: making bread out of plastic. It may come true one day.
Next-generation recycling system that makes plastic use compatible with CO2 reduction
Slide presentation
School of Materials and Chemical Technology
—Encompassing the Disciplines of Science—
Information on School of Materials and Chemical Technology inaugurated in April 2016








