Metal peroxide (MO2, M=Ca, Sr, Ba) is an alternative to hydrogen peroxide (H2O2). It has excellent oxidative properties, superior chemical stability, high purity, and is easy to store and transport. It has been widely used in wastewater treatment and disinfection.
A Chinese research group has recently developed a novel self-cleaning electrode by constructing a micro-/nanostructure of a highly active catalyst with appropriate surface modification, achieving highly stable synthesis of alkaline-earth MO2.
This study was published in Nature Nanotechnology.
The current primary synthesis process of MO2 involves fast decomposition of H2O2, leading to insufficient utilization of H2O2.
In this study, led by Prof. LU Zhiyi at the Ningbo Institute of Materials Technology and Engineering (NIMTE) of the Chinese Academy of Sciences, in collaboration with Prof. JIA Jinping at Shanghai Jiaotong University, the researchers proposed an in situ electrochemical synthesis process to lessen economic losses and reduce explosion risks arising from H2O2 transportation and storage.
High-concentration H2O2 generated by two-electron electrochemical oxygen reduction (2e- ORR) can be efficiently converted into MO2 on the electrode surface. However, severe adhesion of solid MO2 product to the electrode surface can directly shut down the system.
To reduce surface adhesion, the research group constructed a Ni-doped oxygenated carbon electrode with Teflon coating (T-NiOC) as well as a micro-/nanostructure and low surface energy. This greatly reduced the solid-liquid contact area, facilitating rapid detachment of in situ generated MO2 from the self-cleaning electrode surface.
The T-NiOC electrode showed accumulated selectivity of ~99% and stability for over 1,000 hours at a current density of 50 mA cm-2 for electrochemical synthesis of MO2, thus demonstrating broad application potential.
Compared with H2O2, as-synthesized CaO2 performed better in tetracycline degradation with hydrodynamic cavitation (HC).
This work may pioneer and revolutionize other electrochemical solid-state synthesis reactions.
This study was supported by the National Natural Science Foundation of China, the Ningbo Yongjiang Talent Introduction Program, the Ningbo S&T Innovation 2025 Major Special Program, the Bellwethers Project of the Zhejiang Research and Development Plan, and the National Science Foundation of Ningbo, etc.
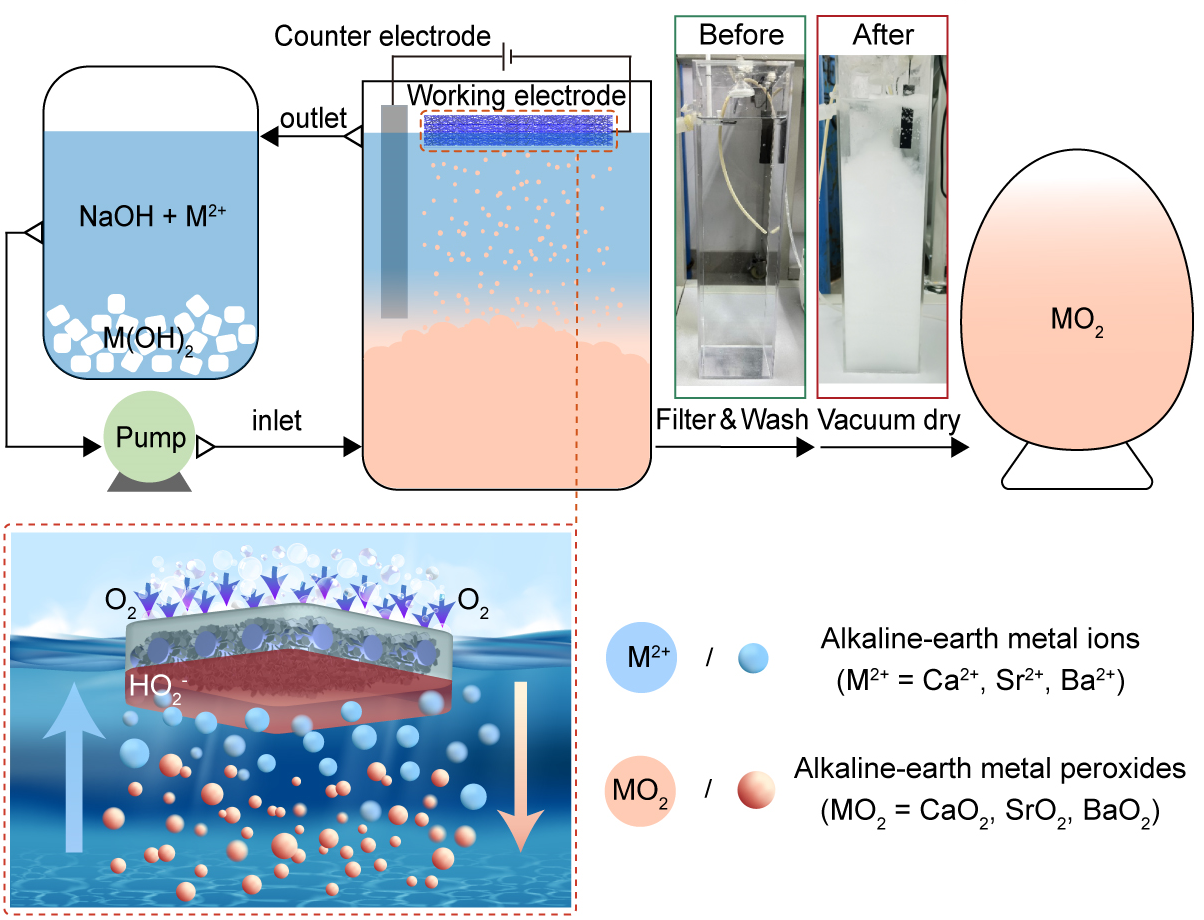
Fig. The in situ electrochemical synthesis process of MO2 (Image by NIMTE)






