A recent study conducted by the Guangzhou Institutes of Biomedicine and Health (GIBH) of the Chinese Academy of Sciences (CAS), in collaboration with the Johns Hopkins University School of Medicine (JHMI), was published in Nature Communications. This study examines the species-specific inhibitory effects of the pluripotency regulator STELLA on UHRF1, an oncogenic driver that is critical for maintaining abnormal DNA hypermethylation in human cancers.
The researchers discovered that human STELLA (hSTELLA) is significantly less effective than mouse STELLA (mSTELLA) at disrupting UHRF1's association with chromatin and its localization within the nucleus. Through structural studies, they identified a region of low sequence homology between these STELLA orthologs, enabling mSTELLA-but not hSTELLA-to bind tightly and cooperatively to the critical histone-binding TTD-PHD domain of UHRF1. This mechanism allows for the specific inhibition of UHRF1 by mSTELLA.
To translate these findings into cancer therapy, the researchers developed a lipid nanoparticle (LNP)-mediated mRNA delivery strategy. They demonstrated that delivering mRNA encoding the "L-shaped" binding conformation of mSTELLA significantly reduces DNA hypermethylation and promotes durable tumor growth inhibition through an epigenetic memory effect. In contrast, delivering mRNA that encodes the "long α-helix" conformation of hSTELLA did not yield similar results.
Dysregulated UHRF1 functions are associated with poor patient prognosis, making it a promising target for cancer treatment. This study not only lays the groundwork for developing mRNA-based DNA demethylation agents but also highlights the potential for the rational design of peptidomimetic or small-molecule mimics of mSTELLA.
This study was supported by the National Natural Science Foundation of China, the Basic Research Project of the Guangzhou Institutes of Biomedicine and Health, the Guangdong Basic and Applied Basic Research Foundation, the National Key R&D Program of China, among others.
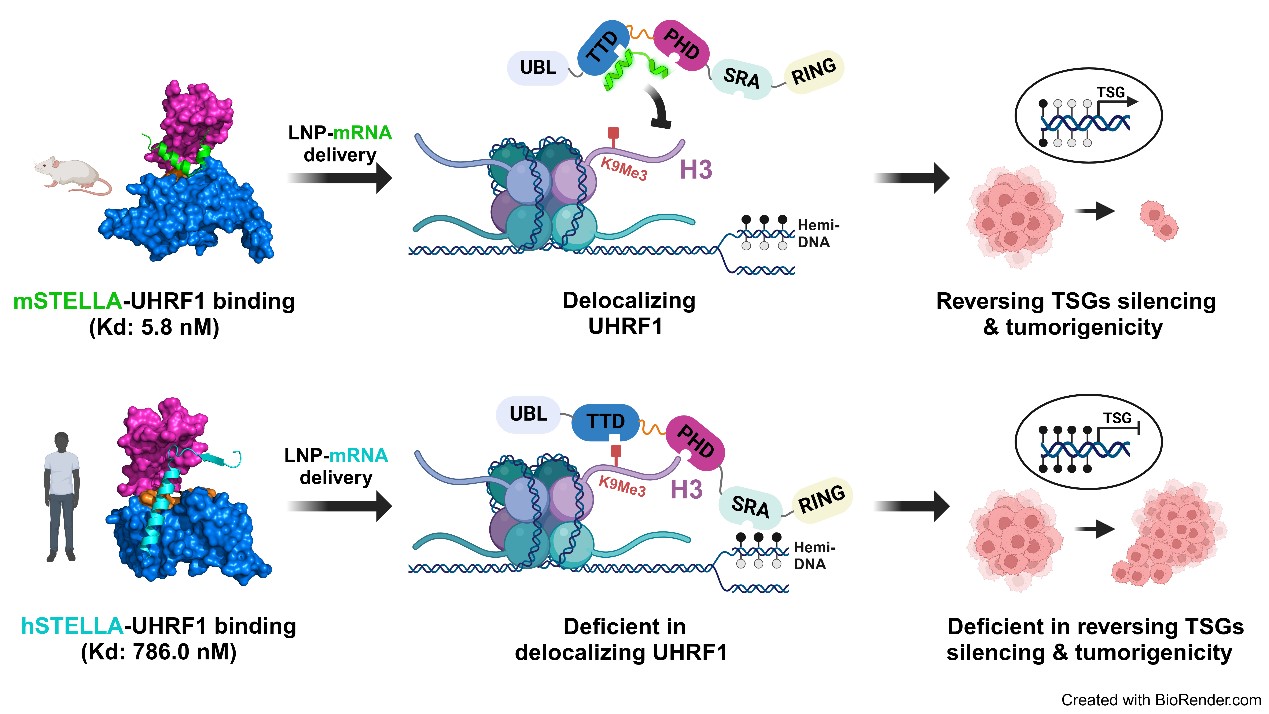
Fig. Ortholog-specific effects of mSTELLA and hSTELLA in inhibiting the abnormal DNA methylation and oncogenic functions of UHRF1 in human cancer cells (Image by Prof. KONG's team)






