According to a research published in Energy Storage Materials recently, a team led by Prof. HU Linhua from Hefei Institutes of Physical Science of the Chinese Academy of Science proposed a general principle through evaluating the highest occupied molecular orbital (HOMO) energy level of molecules and employed it as a critical descriptor to select non-sacrificial anionic surfactant electrolyte additives for stabilizing Zn anodes, realizing sustainable regulation effect with inhibited Zn dendrite growth and side-reactions.
Aqueous zinc-ion batteries (AZIBs) have nowadays stimulated widespread attention for their safety, reliability, and cost-effectiveness. The severe Zn dendrite growth and severe side reactions have become the major roadblock to the widespread commercialization of AZIBs. Anionic surfactants, as a category of typical non-sacrificial additives, have a long history of application in metallurgy as corrosion-inhibiting and deterring agents for Zn plating. Therefore, choosing a suitable anionic surfactant additive promises to fundamentally obtain highly stable and reversible metal anodes.
In this study, the researchers chose three typical anionic surfactants molecules as additives, including sodium dodecyl benzene sulfonate (SDBS), sodium dodecyl sulfonate (SDS), and sodium p-ethylbenzene sulfonate (SEBS) with non-sacrificial behaviors and different HOMO energy levels, and investigated the influence of HOMO energy levels on coordination and adsorption effects for the first time. Experimental and calculational results showed that SDBS, with the highest HOMO energy level, displayed the strongest coordination and adsorption effects, enhancing the stability and reversibility of Zn anode.
Dr. LI Zhaoqian, a member of the research team, highlighted that SDBS with high HOMO energy level "can stop harmful zinc dendrites from growing and make the batteries better at being recharged and reused".
The researchers tested the battery with different materials and found that it worked well with them, even after many cycles.
"The battery worked for over 3200 hours in the test, even at high power levels, which is 30 times longer than with the original electrolyte," said LI.
Researchers assembled Zn//Cu batteries with an average Coulomb efficiency of 98.15% after 800 cycles. Meanwhile, the Zn//NH4V4O10 full battery delivered long-term stability with a capacity retention of 93.5% after 8000 cycles.
This research provides a promising strategy for screening optimal electrolyte additives for high-performance AZIBs and is expected to be applied to other metal batteries.
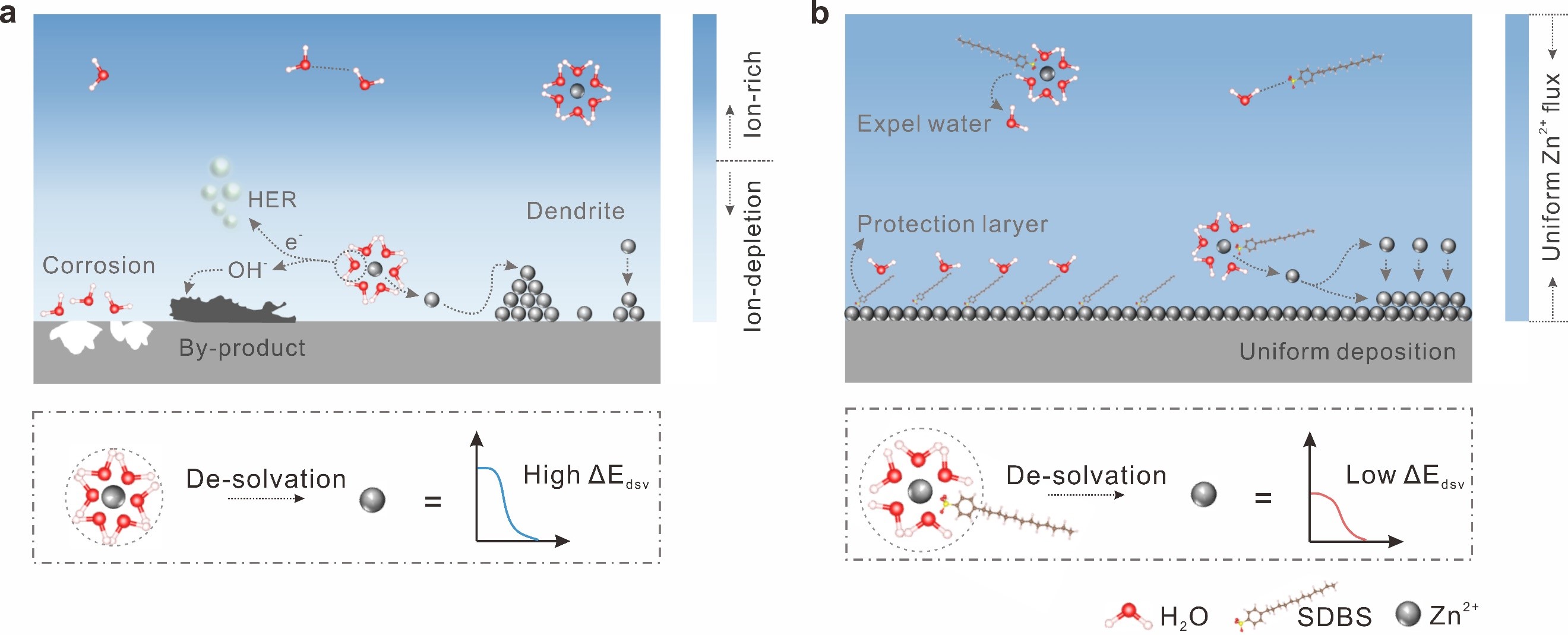
Figure 1. Schematic diagrams for Zn deposition in ZnSO4 (a) and SDBS/ZnSO4 (b) electrolytes. (Image by LI Zhaoqian)






