Key Takeaways
- Researchers have developed a way to engineer pseudo-bonds in a polymer material.
- Their work represents a new way of solidifying materials without relying on permanent chemical bonds.
- Like an epoxy, the material serves as a strong and stable filler-but can also be dissolved and reused, as though untangling a ball of yarn.
Composite adhesives like epoxy resins are excellent tools for joining and filling materials including wood, metal, and concrete. But there's one problem: once a composite sets, it's there forever. Now there's a better way. Researchers have developed a simple polymer that serves as a strong and stable filler that can later be dissolved. It works like a tangled ball of yarn that, when pulled, unravels into separate fibers.
A new study led by researchers at the Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) outlines a way to engineer pseudo-bonds in materials. Instead of forming chemical bonds, which is what makes epoxies and other composites so tough, the chains of molecules entangle in a way that is fully reversible. The research is published in the journal Advanced Materials.
"This is a brand new way of solidifying materials. We open a new path to composites that doesn't go with the traditional ways," said Ting Xu, a faculty senior scientist at Berkeley Lab and one of the lead authors for the study.
Traditionally, there are two ways to make polymer materials strong and tough. In the first, adding a setting agent creates a crosslinked network of polymer molecules held together by permanent chemical bonds. In the second, increasing the length of polymer molecule chains causes them to get more and more entangled, so they can't come apart. The latter, Xu proposed, offers the possibility of a reversible design. She likened the concept to folded proteins that interact without chemical bonds to create sturdy structures in nature, and can later unfold into their constituent strands.
Xu along with her colleagues in Berkeley Lab's Materials Sciences Division wanted to build on this concept and start with a collection of simple polystyrene chains, tangle them together into a tough and stable structure, and then take the material back to its starting point. "Let's say you have a ball of yarn, and it's a mess. You can't untangle it," said Xu. "But if you play with the yarn, maybe you can trick it to untangle."
"This is a brand new way of solidifying materials. We open a new path to composites that doesn't go with the traditional ways."
– Ting Xu
With this in mind, the researchers attached polystyrene chains to hundred-nanometers-diameter silica particles, to create what Xu dubbed "hairy particles." By forming nanocomposites, these hairy particles self-assembled into a crystal-like structure, providing different spaces between each unit for the hairy polymers to fill. The space available to each polystyrene chain depended on its position in the structure-and, therefore, determined how much it tangled together with its neighbors.
By confining the polymer chains into these tiny spaces with different geometries, Xu reduced the freedom with which any cluster of polystyrene chains could move-thus exercising control over how entangled they became. Or, as it turns out, how not entangled: for certain arrangements, the response to squeezing was that a specific cluster of polystyrene chains loosened up in response to an applied force.
"How much entanglement happens with the particles determines their response to an external force," said Xu, who is also a professor in UC Berkeley's College of Engineering and College of Chemistry. By adjusting the polystyrene chain size, as well as precisely where and how many chains were affixed to each facet of the silica particle, she could tweak how the structure responded to dissipate external stresses. Ultimately, these parameters provided the key to engineering entanglement-based "pseudo bonds."
Microscopy studies revealed that while some chains became rigid under confinement, others ultimately disentangled and stretched to dissipate the external stress. The result was a strong, tough, thin-film material, held firmly together by pseudo bonds of tangled polystyrene chains. Adding small amounts of polystyrene chains themselves to the nanoparticle assemblies increased the final load-bearing properties by another 50%.
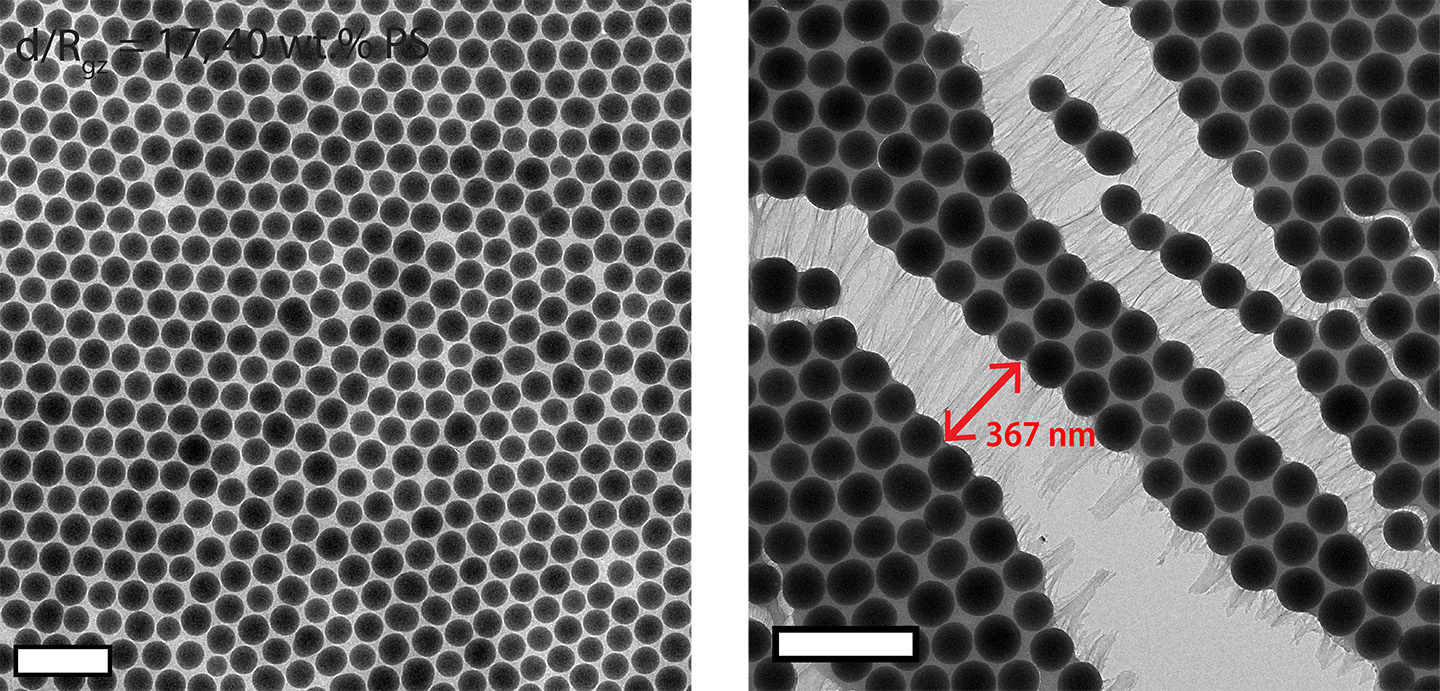
"We were really excited that now we can maneuver amorphous polymer organization using nanoconfinement," said Xu. Until now, amorphous polymers are often randomly entangled, whereas proteins fold nicely. The variations in polystyrene chain arrangement now hits a sweet spot that can be used to engineer composites in a smart way. Moreover, adding a drop of solvent and stirring dissolved the nanocomposite back into its constituent particles suspended: there were no chemical bonds to break, allowing the materials to be reprocessed.
According to Xu, the Berkeley Lab study can readily be extended to other polymers and fillers. Polystyrene is one of the most common polymers and silica is a cheap nanoparticle; nonetheless, Xu hypothesizes that the results will apply to other composites as well. She imagines a future with particles that have other optical or magnetic properties, for example, to create composites for optoelectronic devices. "We can have both strength and toughness, just by modulating how the polymers are distributed," said Xu.
This research used resources at three DOE Office of Science user facilities at Berkeley Lab: the Advanced Light Source, the Molecular Foundry, and the National Energy Research Scientific Computing Center. The work was supported by the Department of Energy's Office of Science (BES).






