T cells - immune cells that patrol our bodies in search of trouble - have become a central focus for UC San Francisco scientists working on living cell therapies, an approach that views cells themselves as a form of medicine.
"From my perspective there's no more important system in the body than the immune system, especially T cells," said Jeffrey Bluestone, PhD, an emeritus professor of medicine at the UCSF Diabetes Center and CEO of the T cell therapy company Sonoma Biotherapeutics. "T cells are potent, diverse and circulate in every tissue from your head to your toes. They play a fundamental role in maintaining a healthy human body."
T cells are potent, diverse and circulate in every tissue from your head to your toes. They play a fundamental role in maintaining a healthy human body.
T cells actually include a diverse group of cells with specialized roles. So-called killer T cells attack foreign invaders, while helper T cells release signals to orchestrate the overall immune response. Once a threat is neutralized, another subset - the regulatory T cells, produce anti-inflammatory factors that shut down the immune response.
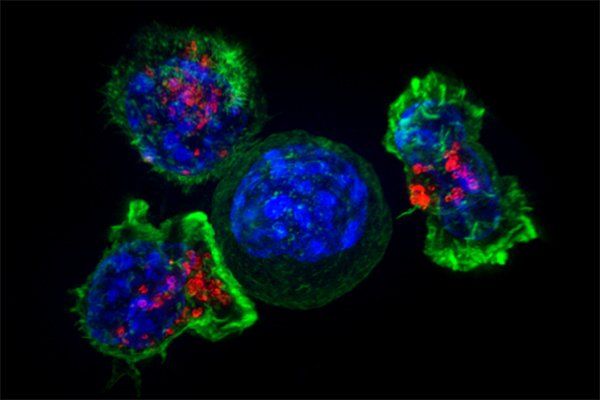
A group of killer T cells (green and red) surrounding a cancer cell (blue, center). Image by NIH
T cells' range of behaviors and their ability to survive for years in our bodies have made them attractive candidates for living cell therapies. UCSF researchers are finding ways to modify T cells to enhance our immune response against cancers and viral infection and to quiet our immune response in autoimmune disorders. Harnessing the power of the body's own systems to create adaptive therapeutics is at the core of the University's new Living Therapeutics Initiative, which will provide new facilities, resources and leadership in this area.
"The therapies we're used to use 'dumb' drugs, with just one trick," said Qizhi Tang, PhD, associate professor of surgery and director of the Transplantation Research Lab. "Living cell therapies can be thought of as smart drugs - they go where they need to go and pull out a variety of mechanisms to deal with the situation. And T cells have an arsenal of tools built in."
Enhancing the Attack
In recent years, scientists have equipped killer T cells with genes for special receptors that match with specific molecules, known as antigens, on cancerous cells, turning their natural seek-and-destroy function against cancers. T cells taken from a patient are modified in the lab and then infused back into the body. The approach, called CAR-T, has worked wonders for blood cancers but have not been effective against solid tumors, which are harder to distinguish from healthy tissue. For example, physicians haven't yet been successful in using traditional CAR-Ts against aggressive brain cancers, known as glioblastomas, in part because common glioblastoma antigens are also found on healthy, non-brain tissue such as in the liver and kidney.
To make "smarter," more discerning CAR-T cells, UCSF researchers have pioneered ways to "program" basic computational abilities into T-cells. For glioblastomas, UCSF researchers are programming CAR-T cells to mount an attack against the target antigen only when they've first detected they are in the brain. These smart cells can safely circulate the rest of the body with no risk to normal tissue.
Such next generation CAR-T cells - developed by Wendell Lim, PhD, chair and Byers Distinguished Professor of cellular and molecular pharmacology, Hideho Okada, MD, PhD, the Kathleen M. Plant Distinguished Professor of neurological surgery, and Kole Roybal, PhD, associate professor of microbiology and immunology, among others - have shown promise in various models of difficult-to-treat cancer, including glioblastomas, ovarian cancer and breast cancer.
With adequate funding, these new CAR-T cells could be tested in glioblastoma patients in just a couple of years, according to Okada, an expert in brain cancers. "This science is ready to move toward clinical trials," he said.
Combined with recent advances in CRISPR gene editing, T-cell therapeutics have the potential to treat even some of the most obscure diseases. For example, UCSF scientists and members of the Weill Neurohub are engineering T cells to go after the virus behind progressive multifocal leukoencephalopathy, a rare but fatal neurological disease. CRISPR, a fast, affordable and precise gene editing tool, has allowed the researchers to test many tweaks to human T cells to find the ideal programming T cells need to fight the virus. Theoretically, the efficiency of CRISPR could allow scientists to customize T cells to target a diverse range of human diseases.
"With CRISPR we can actually take T cells and reprogram them in very targeted and precise ways to make those cells into a new type of cellular medicine," said Alexander Marson, MD, PhD, director of the Gladstone-UCSF Institute of Genomic Immunology, who is part of the effort to treat progressive multifocal leukoencephalopathy with T cells .
Quieting Autoimmunity
Whereas killer T cells attack, regulatory T cells help pump the brakes to stop the immune system from turning against our own healthy tissue. Regulatory T cells employ more than a dozen mechanisms that suppress the activation and function of overactive immune cells. A break down in this function can lead to autoimmune diseases.
Human regulatory T cells are shown in culture. Image by Qizhi Tang, PhD
In 2004, Tang, Bluestone and colleagues discovered that transferring healthy regulatory T cells to mice with autoimmune-driven diabetes could cure the mice of the disease. (In type 1 diabetes, ineffective regulatory T cells are thought to be the root cause of the autoimmune destruction of insulin-producing beta cells of the pancreas.) That finding inspired the Regulatory T Cell Therapy program at UCSF, now "by far the most active regulatory T cell therapy program in the world," said Tang. The program now has 10 clinical trials underway in autoimmune diseases like diabetes and lupus and in mitigating the risk of transplant rejection by the immune system.
Researchers are even looking into whether regulatory T cells could help treat COVID-19, whose deadly symptoms are driven by an out-of-control inflammatory response by the immune system.
"UCSF is already a world leader in T cell therapy research," Marson says. "And bringing all these disciplines together in this collaborative environment means the pace of discovery is moving faster than any of us could have imagined."






