Epilepsy, a neurological condition that results in seizures, is highly prevalent and affects about 1.2% percent of the U.S. population. Worldwide, it affects tens of millions of people, but although its etiology is known for some, the cause is not always clear for others. Incoming Assistant Professor of Pharmacology Quynh Anh Nguyen sat down with us to discuss the results of her most recent paper in which she identified a new brain region associated with certain forms of epilepsy and in which she suggests that targeting that region for treatment may help some patients control their epileptic seizures.

The research, published in Nature Medicine, was spearheaded by Nguyen and neurosurgery resident Ryan Jamiolkowski under the tutelage of Vivek P. Buch and Ivan Soltesz, both faculty members in the Department of Neurosurgery at the Stanford University School of Medicine.
What issue/problem does your research address?
Epilepsy is one of the most prevalent neurological disorders in the world, with tens of millions of people burdened by the occurrence of chronic spontaneous seizures. About one-third of epilepsy patients do not achieve adequate seizure control with existing anti-seizure medications and often elect to undergo invasive surgical resection or ablation of their epileptic brain tissue.
For patients with mesial temporal lobe epilepsy, the most common form of drug-resistant epilepsy, the main surgical target has been the anterior hippocampus and amygdala. About one-third of patients who undergo surgical intervention still do not receive adequate seizure freedom. Thus, there is a critical need to identify novel targets for controlling seizures in this significant patient population.
What was unique about your approach to the research?
This research took a unique approach that bridged the realms of biochemical and molecular tool development, neuroscience basic research, and clinical care.
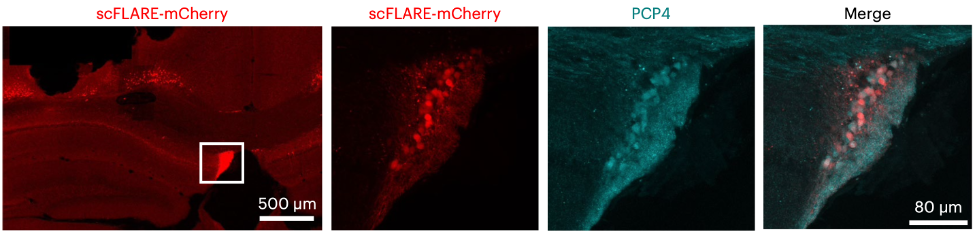
As a postdoc in Ivan Soltesz's lab in the Department of Neurosurgery at Stanford University, I had worked alongside Alice Ting's lab in the Departments of Genetics, Biology, and Chemistry at Stanford to develop a light- and calcium-gated molecular integrator that could be used to label neurons that were active during a designated temporal window. I used this tool to label neurons during seizures in mice, which allowed me to identify a novel brain region involved in epilepsy.
Around the time that I made this discovery, a neurosurgery resident, Ryan Jamiolkowski, had joined the Soltesz lab and shared with me that he had an epilepsy patient whose seizures seemed to come from this region. We put forth a combined effort to show that this region is an important seizure node and a viable target for intervention in both mice and humans with epilepsy.
Overall, within the span of about 2.5 years, we took a basic science molecular tool and used it to make a fundamental discovery that could directly impact patient care. As a new assistant professor at Vanderbilt, I will continue this interdisciplinary approach in my own lab, with basic scientists and clinicians working side by side to find ways to help improve the human condition.
What were your findings? How can non-expert readers understand their significance?
We found that an understudied region of the brain called the fasciola cinereum is highly active during seizures in both mice and humans with epilepsy. Considering that most clinical targets focus on the anterior hippocampus and that the FC is located in the posterior hippocampal tail, the FC has previously been overlooked as a potential site of seizure onset. Yet, when we inhibited the activity of the neurons in this region in mice specifically during seizures, we found a significant reduction in seizure duration.
In all six of our human epilepsy patients, we found that the FC was involved in most of their seizures. In a patient with previously uncontrolled epilepsy who had undergone a prior surgical ablation of their anterior hippocampus, we found that targeted lesioning of this region reduced their seizure burden by 83 percent.
Together, these results highlight the involvement of the hippocampal tail in epilepsy and suggest a fundamental change in the standard of care to include the FC for consideration as a potential target site of seizure localization.
What do you hope will be achieved with the research results on the short term?
In the short term, I hope that more clinicians will consider the FC region as a candidate site for seizure localization in their patients with epilepsy. In addition, my own lab will be focusing on characterizing the neurons in this region and their function both in the normal as well as epileptic conditions. Although more work is needed to determine whether targeting FC alone is a viable intervention in humans, more patient recordings of FC and further basic science characterization of FC will help shed light on this understudied brain region and how it goes awry in disease.
What are the long-term societal, environmental, or economic benefits of this research?
This research has opened new avenues of investigation into the FC region, both from a clinical as well as basic science perspective. There is much to be done to understand what this region is, how it functions normally, and how its dysfunction leads to disease. In addition, we don't know whether this area is also involved in other types of neurological disorders.
We hope this research will lead to larger-scale clinical trials on FC as a potential target to treat uncontrolled seizures. This may fuel further development of novel approaches or tools to specifically modulate this region in humans. Together, these efforts may lead to a significant reduction in the proportion of patients with uncontrolled epilepsy.
Where is this research taking you next?
A central focus of my new research lab will be to uncover the basic characteristics and functions of the FC and how these properties are affected by and lead to neurological disorders such as epilepsy. I will continue to work with my collaborators at Stanford and the University of Cambridge to develop new tools that will help me study this region, and with clinicians both at Stanford and at Vanderbilt who can help translate my basic science findings from mice to humans.
GO DEEPER
The article "The Fasciola Cinereum of the Hippocampal Tail as an Interventional Target in Epilepsy" was published in Nature Medicine in April 2024.
FUNDING AND ACKNOWLEDGEMENTS
This study was funded by the Stanford Maternal & Child Health Research Institute, the LGS Foundation, the Stanford University School of Medicine, the Wellcome Trust, and the National Institutes of Health. The authors also appreciate help and materials from T. Takemori and A. Ishige at RIKEN, A. Ting at Stanford University, and C. Porter and U. Chon.






