A research team led by Prof. ZHANG Guangjin from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences has fabricated a novel InOOH electrocatalyst with unique frustrated Lewis pairs (FLPs) for efficient urea synthesis at ambient conditions.
This work was published in Chem Catalysis on Dec. 15.
The industrial process of nitrogen (N2) fixation, i.e., the amino synthesis process, consumes a great deal of energy and produces a large amount of carbon dioxide (CO2) due to harsh reaction conditions.
Electrochemical C-N coupling reactions at ambient conditions can realize both N2 fixation and CO2 conversion into value-added urea molecules, thus solving the problem of excessive CO2 emissions during the N2 fixation process. However, this strategy remains challenging due to the low catalytic activity and selectivity of the catalyst.
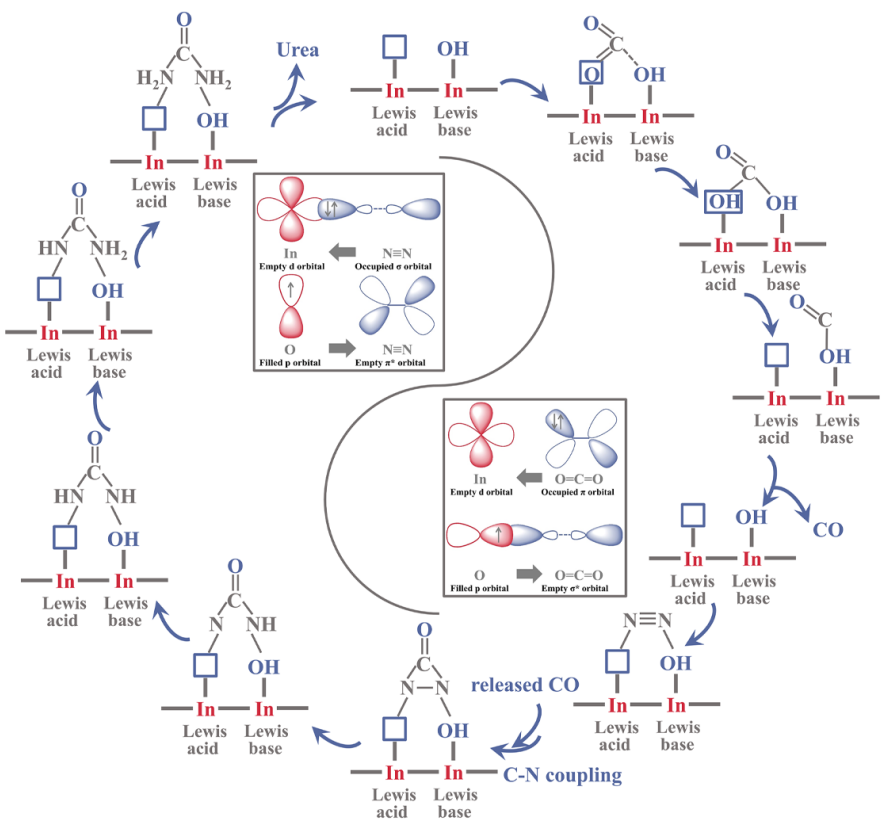
FLPs are composed of a Lewis acid and a Lewis base that are sterically prevented from bond formation. "FLPs possess the capability of chemisorbing and reacting with various gas molecules. They can capture and react with N2 and CO2, thus forming a new strategy for urea electrosynthesis," said Prof. ZHANG.
In this study, the researchers synthesized rice-like InOOH nanoparticles coupled with well-defined FLPs (i.e., In···In-OH), thus achieving a urea yield rate of 6.85 mmol h-1 g-1.
The electron-deficient Lewis acidic In sites and electron-rich Lewis basic In-OH achieved the targeted chemisorption of the N2 and CO2 molecules, respectively, by electronic interaction.
The bonding and antibonding orbitals of reactant molecules interacted with the unoccupied orbitals of the Lewis acid and nonbonding orbitals of the Lewis base to generate desired intermediates for urea synthesis in artificial FLPs.
The researchers used linear sweep voltammetry to preliminarily evaluate the potential performance of urea electrosynthesis with IOOH hybrids.
The results showed that InOOH hybrids exhibited good performance in the electrocatalytic nitrogen reduction reaction and the CO2 reduction reaction, thus feasibility of the electrocatalytic urea production process.