The traditionally cumbersome yet widely-used Birch reduction can now be carried out in a mere minute in air using an optimized mechanochemical approach.

Ball milling jar and reagents used in the simplified mechanochemical Birch reduction. (Photo: Koji Kubota)
The Birch reduction is a reaction commonly used to make medicines and bioactive compounds, but the laborious process typically requires that chemists handle liquid ammonia, use cryogenic temperatures, and carry out time-consuming steps. Researchers at the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD) in Hokkaido University have developed a simplified method for performing the Birch reduction that avoids the use of ammonia, can be done at room temperature and in ambient air, and is 20-150 times faster than conventional methods.
A number of lithium-based methods for performing the Birch reduction in solution have been previously developed, but since lithium reacts with both air and water, these processes still required complicated reaction setups with an inert atmosphere or dehydrated conditions. Researchers in this study saw an opportunity to avoid these issues by switching from a solution-based method to a solvent-less method using a ball mill, in which reactants are shaken rapidly in a small metal jar along with a metal ball that smashes the solid reactants together.
"In previous studies, we found that using a ball mill for reactions of metals such as magnesium and calcium with organic compounds improved the reaction rate and greatly simplified the process," said co-author Associate Professor Koji Kubota. "Based on this, we wondered if we could develop a more straight-forward Birch reduction process by performing reactions of lithium metal with aromatic compounds in a ball mill."
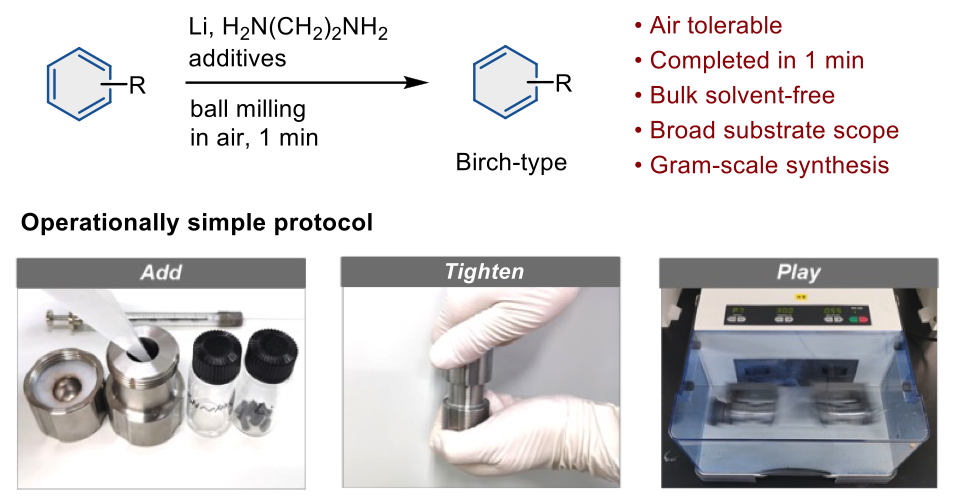
Overview of the simplified protocol for the Birch reduction using a ball mill. (Yunpeng Gao, Koji Kubota, Hajime Ito. Agewandte Chemie International Edition. March 21, 2023)
The key to this strategy is that the mechanical impact from the ball breaks through the surface layer on the lithium that reacted with the air, exposing the pure lithium underneath to the other reactants and enabling the Birch reduction to proceed. This approach can be carried out in ambient air and at room temperature, making for a much easier process.
Researchers demonstrated the versatility of the process, successfully testing it with a wide variety of organic compounds, including pharmaceutical intermediates and other bioactive molecules. In most cases, the Birch reduction was completed in an astonishingly quick one minute.
The process was successfully scaled up to larger gram-scale batches, and the team believes this technique could enable the simplified synthesis of a wide variety of molecules, while also marking an important advance in mechanochemistry.
"The Birch reduction is used extensively in drug discovery and various chemical industries, and our research has made significant advancements, resulting in a much simpler and more eco-friendly Birch reduction process," commented Professor Hajime Ito, who led the study. "We expect this breakthrough to accelerate drug discovery and various other areas of chemical research."

Associate Professor Koji Kubota (left) and Professor Hajime Ito (right) of the research team at the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University. (Photo: WPI-ICReDD)
Original Article:
Yunpeng Gao, Koji Kubota, Hajime Ito. Mechanochemical Approach for Air-Tolerant and Extremely Fast Lithium-Based Birch Reductions in Minutes. Angewandte Chemie International Edition. March 21, 2023.
DOI: 10.1002/anie.202217723
Funding:
This work was financially supported by the Japan Society for the Promotion of Science (JSPS) via KAKENHI grants 22H00318, 21H01926, 22K18333, and 22H05328; the Japan Science and Technology Agency (JST) via CREST grant JPMJCR19R1 and FOREST grant JPMJFR201I; and the Institute for Chemical Reaction Design and Discovery (ICReDD) established by the World Premier International Research Initiative (WPI), MEXT, Japan.






