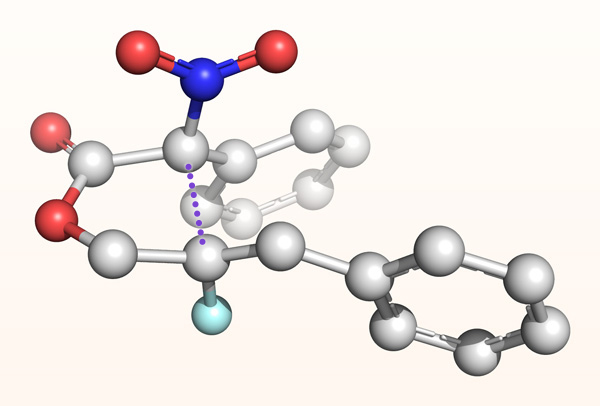
Figure 1: A computed molecular structure showing an intermediate structure formed during the uphill formation of a bond between two neighboring tetrasubstituted carbon atoms (purple dotted line: a newly forming bond; white spheres: carbon atoms; red spheres: oxygen atoms; blue sphere: nitrogen atom; cyan sphere: fluorine atom). © RIKEN
Complex molecules with promising biomedical properties can be made by using a two-step process in which the second step is downhill from an energy point of view, RIKEN chemists have shown1. They demonstrated the potential of this approach by using it to assemble organic molecules with potent bioactivity against fungi.
Making biomedically useful compounds often entails creating bonds that are energetically demanding to form.
In stable organic molecules, each carbon atom has four bonds with other atoms. In general, if there are more bonds with small hydrogen atoms, the environment around the carbon atom is less crowded, making it easier for new carbon-carbon bonds to form.
Conversely, the hardest carbon-carbon bonds to make are those that result in two adjacent 'tetrasubstituted' carbon atoms that don't include a single hydrogen atom (Fig. 1).
A team of researchers led by Mikiko Sodeoka from the RIKEN Center for Sustainable Resource Science has focused on these challenging types of carbon-carbon bonds, which form the backbone of organic molecules.
The team has found that the entire sequence can be driven forward by incorporating challenging bond-forming steps into a two-step process in which the first step is 'uphill', requiring the input of energy, while the second step is 'downhill', releasing energy.
"Our aim is to expand our reaction-design concept based on an energetic uphill and downhill sequential process," explains Yoshihiro Sohtome, a member of Sodeoka's team.
For the energetically uphill part of their sequential process, the team selected a copper-catalyzed, radical-based reaction for generating a bond to form adjacent tetrasubstituted carbons.
"This bond formation is believed to be very difficult, because the crowded resulting bond is reversibly cleavable," says Sohtome. "We sought to address this problem by including an energetically downhill second step in the sequence that prevents the bond from being cleaved."
Adding to the complexity of the transformation, the radical termination step was designed to involve the formation of a new carbon-oxygen double bond, with the oxygen coming directly from oxygen molecules in the air.
The strategy successfully delivered the crowded carbon-carbon bond and the carbon-oxygen double bond, the team showed. "Our approach simultaneously solved two different and challenging problems," says Sohtome.
Among the molecules that the team synthesized using this approach was a family of γ-lactones, which displayed potent growth inhibition against the fungus brewer's yeast.
The strategy is still in the proof-of-concept stage, Sohtome notes. "However, we strongly believe that providing novel molecular structures using our catalyst has great potential to explore not only antifungal agents but also other bioactive molecules," he says.
The team is also exploring other classes of transformation based on the uphill/downhill bond-forming process.






