Nickelates are a material class that has excited scientists because of its recently discovered superconducting ability, and now a new study led by Cornell has changed where scientists thought this ability might originate, providing a blueprint for how more functional versions might be engineered in the future.
Superconductivity was predicted in nickel-based oxide compounds, or nickelates, more than 20 years ago, yet only realized experimentally for the first time in 2019, and only in samples that are grown as very thin, crystalline films - less than 20 nanometers thick - layered on a supporting substrate material.
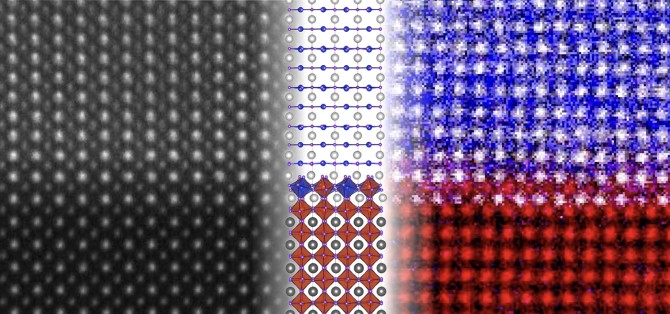
Researchers at Cornell used advanced electron microscopy techniques to uncover an unexpected atomic structure within a newly discovered class of nickel-based superconductors.
Researchers worldwide have been working to better understand the microscopic details and origins of superconductivity in nickelates in an effort to create samples that successfully superconduct in macroscopic "bulk" crystalline form, but have yet to be successful. This limitation led some researchers to speculate that superconductivity was not being hosted in the nickelate film, but rather at the atomic interface where the film and substrate meet.
In "Resolving the Polar Interface of Infinite-Layer Nickelate Thin Films," published March 27 in Nature Materials, a research team led by Lena Kourkoutis, associate professor of applied and engineering physics, and Berit Goodge, Ph.D. '22, Minerva Group leader at the Max Planck Institute for Chemical Physics of Solids, used an advanced scanning transmission electron microscope and electron energy loss spectroscopy to provide an unprecedented look at the atomic interface between a nickelate film and its strontium titanate substrate.
What they discovered was an intermediate compound formed at the interface - one that had never been predicted and that alleviated the kind of electronic charge buildup that could give rise to superconductivity. The finding proved that the previously proposed model of a superconducting interface is not valid - the superconductivity is instead occurring in the nickelate film itself.
"An important outcome of our work was establishing what the atomic and electronic structure of the interface actually looks like," Kourkoutis said. "Previous work had assumed a structure that, as far as we can see, simply doesn't exist. We are now providing the true structure of that interface so that scientists can use that information to better understand these materials and engineer new variations in the future."
The discovery is encouraging for the field because it shows that superconductivity may not be reliant on the thin-film geometry. This means that creation of superconducting bulk crystals theoretically should be possible, according to Goodge.
"There are certain experiments to probe the fundamental physics of this system which can't be done until we have really high-quality bulk crystals," Goodge said. "And so, while the thin films are an excellent platform, we do want to be able to expand out to larger crystals to do those other measurements. Now we know there's not some fundamental physical limitation why superconductivity occurs only in thin films."
Goodge added that these efforts will support advancements across a broader range of superconducting families to develop new compounds for technological applications.
The research conducted at Cornell was supported by the Department of Defense Air Force Office of Scientific Research, the Packard Foundation and the National Science Foundation. Co-authors include scientists at Stanford University, the University of Duisburg-Essen, and City University of Hong Kong.
Diane Tessaglia-Hymes is a communications specialist for Cornell Engineering.