Metal-organic frameworks, or MOFs, are composed of metal ions periodically surrounded by organic bridging molecules, and these hybrid crystalline frameworks feature a cage-like hollow structure. This unique structure motif offers great potential for a range of applications in energy storage, chemical transformations, optoelectronics, chemiresistive sensing, and (photo)electrocatalysis, among others. Debuted in the early 2000s, MOFs are a fascinating nanomaterial. Though numerous applications exploit MOFs, little has been known as to how oxygen may work in the synthesis of MOFs.
An international team of chemists, led by Distinguished Professor Rodney S. Ruoff (Department of Chemistry) and senior chemist Dr. Yi Jiang, from the Center for Multidimensional Carbon Materials (CMCM), within the Institute for Basic Science (IBS) at UNIST, has identified how oxygen affects the synthesis of a novel MOF; copper 1,3,5-triamino-2,4,6-benznetriol metal-organic framework [Cu3(TABTO)2-MOF]. This research has been carried out in collaboration with their colleagues at UNIST and Sungkyunkwan University (SKKU).
"Since organic redox-active ligands are usually sensitive to oxygen, the presence of oxygen is not favored in many organic reactions. However, oxygen can be helpful for the synthesis of some redox-active ligand-based MOFs, but many chemists did not realize this," notes Dr. Yi Jiang, the first author of the study. The researchers synthesized a 2D conjugated MX2Y2-type (M = metal, X, Y = N, S, O, and X ≠ Y) Cu3(TABTO)2-MOF based on a redox-active ligand (1,3,5-triamino-2,4,6-benzenetriol). The role of oxygen in the synthesis of this MOF was identified by comparing the results from experiments in air and inert gas (argon): Pure Cu3(TABTO)2-MOF was produced in the presence of oxygen, but the Cu3(TABTO)2-MOF together with copper metal was formed if oxygen was absent. Dr. Jiang adds, "Our study suggests that oxygen prevents these ligands from reducing the Cu (I and II) ions to Cu metal, facilitating the synthesis of a pure MOF."
They also revealed that Cu3(TABTO)2-MOF became electrically conductive after being chemically oxidized by iodine because of the formation of CuI and carriers. It is originally an insulator with almost no electrical conductivity. The iodine-doping generates 0.78 siemens per centimeter of electrical conductivity in the Cu3(TABTO)2-MOF pellet that was synthesized in air. Further experiments and analysis found the metallic characteristics of the materials.
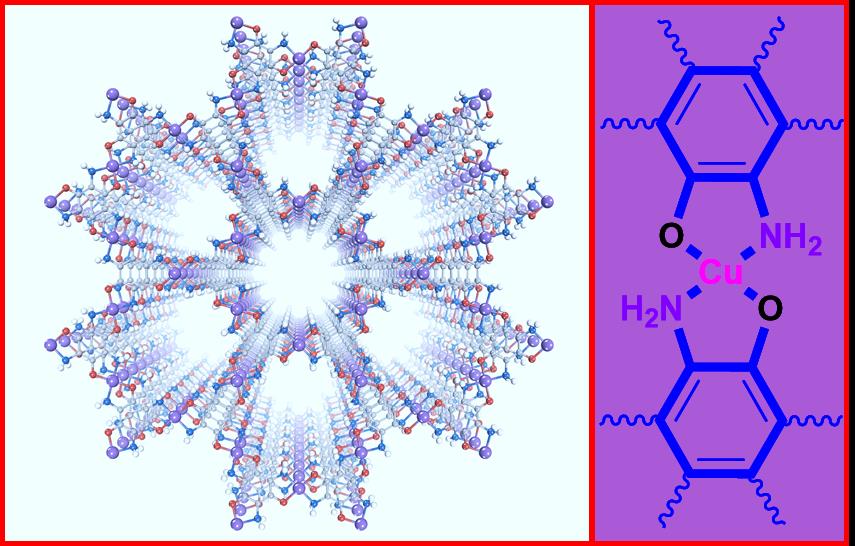
▲ Figure 1: The structure of the Cu3(TABTO)2-MOF (carbon, nitrogen, oxygen, hydrogen, and copper atoms are gray, blue, red, white, and purple, respectively).
Modelling the structure via detailed density functional theory (DFT) calculations, the researchers also experimentally studied the structure of this 2D MOF through X-ray diffraction, diffuse reflectance UV-vis, X-ray photoelectron, electron paramagnetic resonance, and Raman spectroscopies.
"Our work contributed to a fundamental understanding of the role of oxygen in the synthesis of redox-active ligands-based MOFs, and should inspire the community to pay more attention to the role oxygen can play in synthesis of redox-active ligands-based MOFs," says Director Rodney S. Ruoff, the corresponding author of the study. Dr. Jiang further explains, "Most work in this field focused on the synthesis of MX4-type (M = metal, X = N, O, or S) MOFs based on redox-active ligands. The synthesis of new electrically conductive MOFs that are not the MX4-type is both challenging and meaningful work. Both the as-synthesized and iodine-doped Cu3(TABTO)2-MOFs might be useful in catalysis and energy-related applications."
UNIST and SKKU collaborators measured physical properties and did theoretical modeling of this new MOF. Professor Jung-Woo Yoo and his student Inseon Oh (Department of Materials Science and Engineering, UNIST) studied the iodine doping-induced electrical conductivity of the Cu3(TABTO)2-MOF. The metallic characteristics of this iodine-doped material were also explored by Professor Jungseek Hwang and his Postdoctoral Fellow Dr. Yu-Seong Seo (Department of Physics, SKKU). Professor Sang Kyu Kwak and his student Se Hun Joo (School of Energy and Chemical Engineering, UNIST) modeled the structure through detailed DFT calculations. This research was supported by IBS, UNIST, Korea Institute of Science and Technology Information (KISTI), and the National Research Foundation of Korea.
Rodney S. Ruoff
Distinguished Professor, School of Natural Sciences, UNIST
Director, IBS Center for Multidimensional Carbon Materials (CMCM)
Dahee Carol Kim
Public Information Officer
T: +82-42-878-8133
Story Source
Materials provided by Institute of Basic Science.






