Direct splitting: electrochemical process uses carbon dioxide to produce oxygen
To mitigate global climate change, emissions of the primary culprit, carbon dioxide, must be drastically reduced. A newly developed process helps solve this problem: CO2 is directly split electrochemically into carbon and oxygen. As a Chinese research team reports in the journal Angewandte Chemie, oxygen could also be produced in this way under water or in space-without requiring stringent conditions such as pressure and temperature.
© Wiley-VCH, re-use with credit to 'Angewandte Chemie' and a link to the original article.
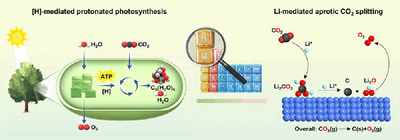
Leafy plants are masters of the art of carbon neutrality: during photosynthesis, they convert CO2 into oxygen and glucose. Hydrogen atoms play an important role as "mediators". However, the process is not particularly efficient. In addition, the oxygen produced does not come from the CO2 but from the absorbed water. True splitting of CO2 is not taking place in plants and also could not be achieved at moderate temperatures by technical means so far.
Ping He, Haoshen Zhou, and their team at Nanjing University, in collaboration with researcher from Fudan University (Shanghai) have now achieved their goal to directly split CO2 into elemental carbon and oxygen. Instead of hydrogen, the "mediator" in their method is lithium. The team developed an electrochemical device consisting of a gas cathode with a nanoscale cocatalyst made of ruthenium and cobalt (RuCo) as well as a metallic lithium anode. CO2 is fed into the cathode and undergoes a two-step electrochemical reduction with lithium. Initially, lithium carbonate Li2CO3 is formed, which reacts further to produce lithium oxide Li2O and elemental carbon. In an electrocatalytic oxidation process, the Li2O is then converted to lithium ions and oxygen gas O2. Use of an optimized RuCo catalyst allows for a very high yield of O2, over 98.6 %, significantly exceeding the efficiency of natural photosynthesis. As well as pure CO2, successful tests were also carried out with mixed gases containing varying fractions of CO2, including simulated flue gas, a CO2/O2 mixture, and simulated Mars gas. The atmosphere on Mars consists primarily of CO2, though the pressure is less than 1 % of the pressure of Earth's atmosphere. The simulated Mars atmosphere thus contained a mixture of argon and 1 % CO2.
If the required power comes from renewable energy, this method paves the way toward carbon neutrality. At the same time, it is a practical, controllable method for the production of O2 from CO2 with broad application potential-from the exploration of Mars and oxygen supply for spacesuits to underwater life support, breathing masks, indoor air purification, and industrial waste treatment.
(2677 characters)
About the Author
Dr. Ping He is a Professor and Head of the Department of Energy Science and Engineering at the College of Engineering and Applied Sciences, Nanjing University. His research interests focus on transformative electrochemical energy technologies, including high-energy-density batteries, electrochemical CO₂ reduction, and lithium resource extraction and recycling. He is a Fellow of the Royal Society of Chemistry and serves as an Associate Editor for ACS Energy & Fuels.
Copy free of charge-we would appreciate a transcript/link of your article. The original articles that our press releases are based on can be found in our online pressroom.






