A joint research team, affiliated with UNIST has announced that they have successfully clarified the correlation between oxygen generation and a change in the microstructure of the cathode material of a battery in a high-temperature operating environment.
This breakthrough has been led by Professor Sungkyun Jung in the School of Energy and Chemical Engineering at UNIST, in collaboration with researchers from the Korea Atomic Energy Research Institute and Seoul National University.
In this study, the research team presented a new way of battery design, in which oxygen generation is reduced by an increase in the cobalt content of the cathode material. According to the research team, a phase transition to a rock salt structure causing oxygen generation can be further impeded when the cobalt content is higher.
"Oxygen from the cathode of a battery is the main cause for fire and explosion and contact between oxygen and an organic battery electrolyte in a high-temperature operating environment may result in combustion," noted the research team. "In order to develop a safer battery, it is important to figure out the principle and amount of internal oxygen generation."
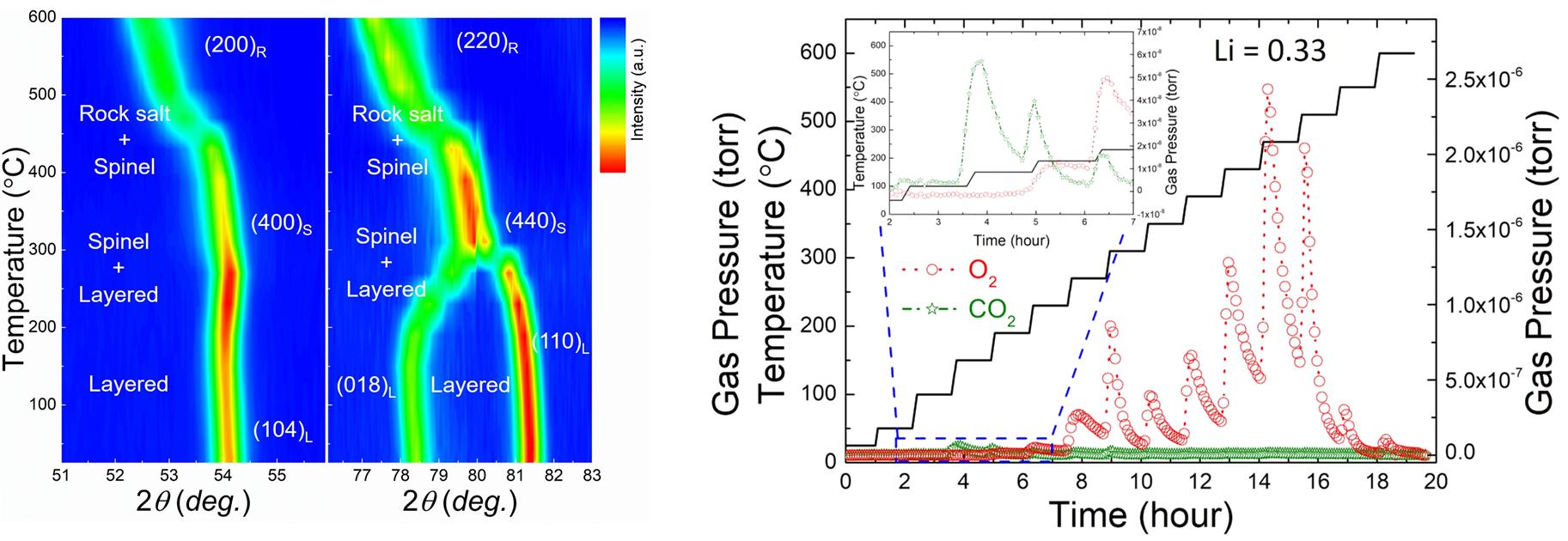
Researchers analyzed oxygen generation and how the internal atom arrangement changes in real-time during an increase in battery temperature. As a result, it confirmed that partial oxygen generation at an electrode results from a phase transition in which the cobalt in the cathode material turns into a spinel structure while moving from a transition metal layer to a lithium layer and a larger amount of oxygen is generated when the spinel structure turns into a rock salt structure with a further increase in temperature.
"According to our experiments, cobalt is capable of impeding the phase transition to the rock salt structure," noted the research team. "The outcome of the research can be cathode material composition and design guidelines for safer batteries."
The findings of this research were made available in December 2021, ahead of its publication in Advanced Functional Materials. This study has been supported by the Center for Nanoparticle Research at the Institute for Basic Science (IBS), the Ministry of Science and ICT (MSIT), and the Samsung Research Funding Center of Samsung Electronics.
Journal Reference
Sung-Kyun Jung, Hyungsub Kim, Seok Hyun Song, et. al., "Unveiling the Role of Transition-Metal Ion in the Thermal Degradation of Layered Ni-Co-Mn Cathodes for Lithium Rechargeable Batteries," Adv. Funct. Mater., (2021).






