The maternal-to-zygotic transition, involving maternal mRNA clearance and zygotic genome activation (ZGA), is a conserved and fundamental process during vertebrate embryogenesis. During this critical developmental period, the embryo undergoes dramatic reprogramming by switching from a maternal factor-dominated state to a zygotic factor-driven state. Due to the transcriptional quiescence of the zygotic genome during the initial developmental stages, selective maintenance and translational control of maternal mRNAs are essential.
Previous studies by Dr. YANG Yungui's lab from Beijing Institute of Genomics of the Chinese Academy of Sciences (China National Center for Bioinformation) and Dr. LIU Feng's lab from Institute of Zoologyof the Chinese Academy of Sciences have illustrated that RNA 5-methylcytosine (m5C) modification and RNA structure promote the maternal-to-zygotic transition (MZT) by selectively protecting the stability of maternal mRNAs. Nonetheless, how fertilization mechanistically leads to MZT is not understood yet.
Recently, a new study led by Dr. YANG Yungui and Dr. LIU Feng demonstrated that Ddx3xb phase separation regulates MZT by promoting maternal mRNA translation, which has been published in Cell Research.
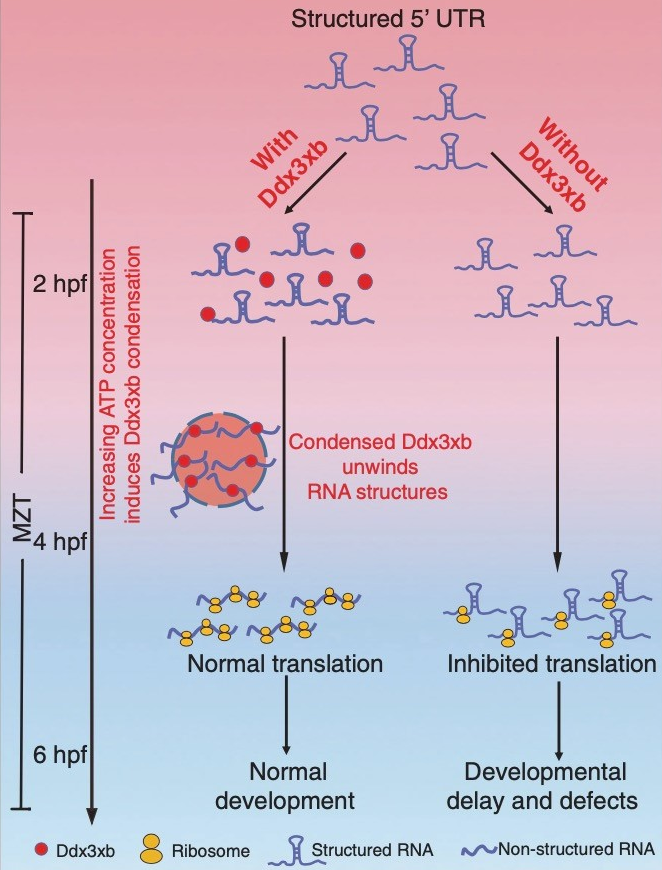
The researchers found that Ddx3xb is highly expressed in zebrafish embryos, and revealed that Ddx3xb phase separation depends on the presence of its N-terminal IDR through a series of phase separation assays. Intriguingly, the researchers observed that endogenous Ddx3xb formed condensates through liquid-liquid phase separation (LLPS) only at 4 hpf, but not at 2 hpf. Since the 4 hpf period after fertilization is a critical time point for MZT, these data may hint a role of Ddx3xb in regulating MZT through LLPS activity. Then, they generated a ddx3xb-null frameshift mutant by CRISPR/Cas9, and found that loss of Ddx3xb induces developmental delay, and LLPS ability of Ddx3xb is essential for zebrafish embryogenesis.
Moreover, the researchers investigated the underlying molecular mechanism by multi-omics sequencing and biochemical experiments. RNA-seq data showed that Ddx3xb phase separation could regulate the degradation of maternal mRNAs and the activation of zygotic genes, indicating that the phase separation activity of Ddx3xb is critical for MZT.
In combination with previously published data about RNA structure in zebrafish embryos, the researchers discovered that 5' UTR structures of Ddx3xb target mRNAs were significantly more open than those of non-Ddx3xb targets at 4 hpf. The results from in vitro helicase assays demonstrated that phase separation, mediated by the N-terminal intrinsically disordered region (IDR) in Ddx3xb, facilitates the Ddx3xb helicase activity. In support of the role of Ddx3xb in controlling translation through unwinding RNA structure in 5' UTR, the researchers further demonstrated that Ddx3xb phase separation could improve the translation efficiency of maternal mRNAs via Ribosome profiling assay.
Overall, this study demonstrates that Ddx3xb phase separation regulates MZT by promoting maternal mRNA translation. These findings expand our understanding of the role of phase separation in biological processes, such as its dynamic regulation of gene expression during MZT.
This research was selected as the Cover Story of Cell Research, and were highlighted in Cell Research by Nadine L. Vastenhouw of the University of Lausanne, Switzerland, entitled "Maternally Loaded RNAs: No Time to Die".