A team led by Prof. Dr. Han Sun from the Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) has elucidated an important mechanism in the function of TREK channels at an atomic level. The results, published in the journal "Nature Communications," could facilitate the development of therapeutics for diseases such as ischemia, epilepsy, and depression.
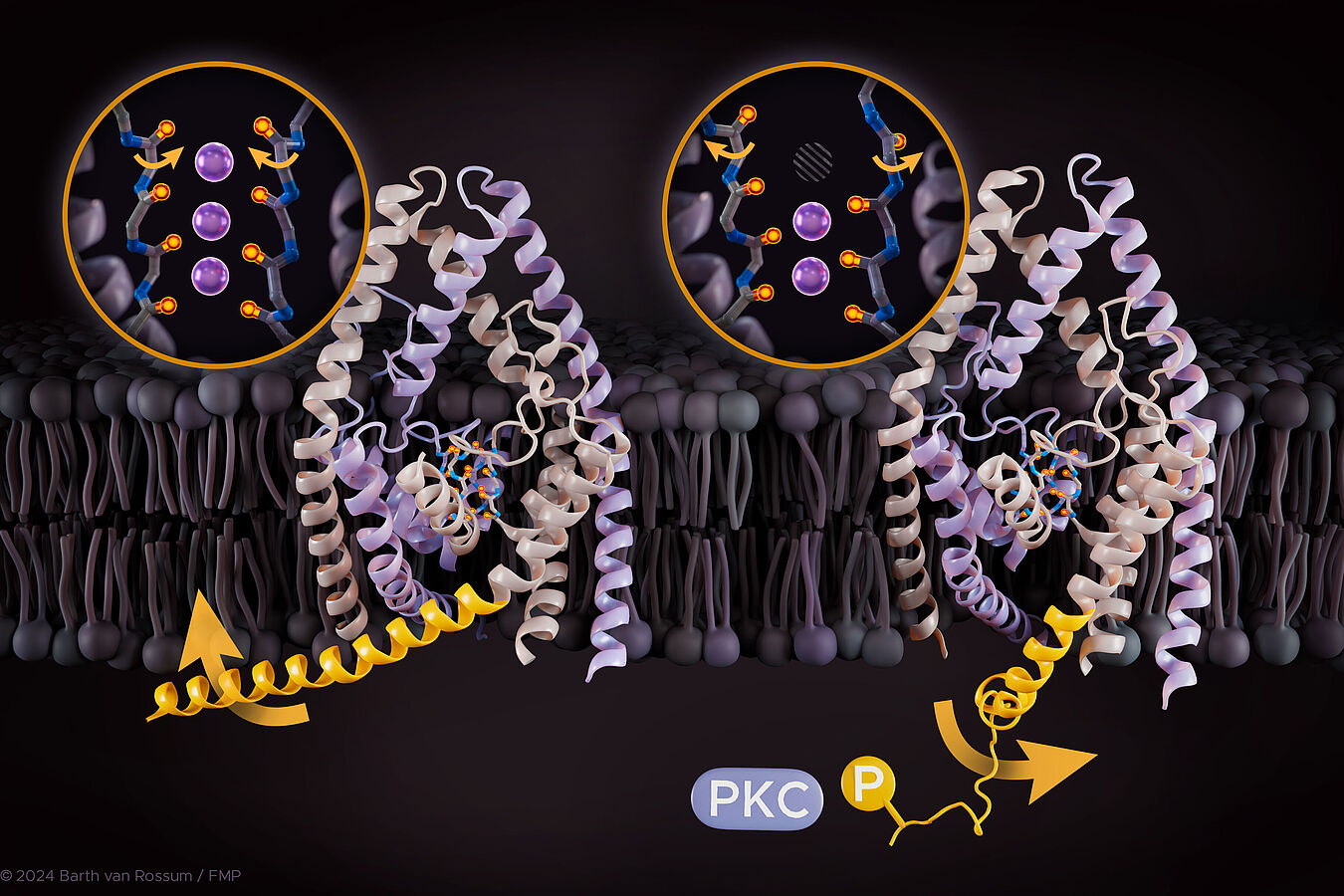
Atomistic mechanism illustrating how TREK K2P channels sense external stimuli and regulate the main gate. | Visualization: Barth van Rossum
The human body is composed of cells where various processes occur constantly. Some of these processes take place across the cell membrane through potassium channels that transport potassium ions. These channels play a central role in vital physiological processes such as regulating cell growth, sensory processes, and neuromuscular excitability.
The current study analyzed TREK channels, which belong to the subfamily of two-pore domain potassium channels. TREK channels are found in the cardiovascular system as well as in the central and peripheral nervous systems. Mutations leading to a loss of function in these channels can cause diseases like ischemia (reduced blood flow), epilepsy, and depression. Therefore, TREK channels are considered promising targets for drug development. Phosphorylation regulated by protein kinases A, C, and G plays a crucial role in the physiological processes and diseases involving TREK channels, such as the development of hyperalgesia under inflammatory conditions and depression. Phosphorylation refers to the attachment of a phosphate group to a protein, a fundamental biochemical process essential for signal transduction, where signals are transmitted through a series of processes and interactions.
"However, it is not yet fully understood how phosphorylation affects the activity of TREK channels, which complicates drug development," says Prof. Dr. Han Sun, head of the research group "Structural Chemistry and Computational Biophysics" at FMP. Together with her team and collaborators from the University of Kiel, who worked on experimental validation, the researchers have now closely examined this mechanism.
Phosphorylation and other external stimuli are recognized by the TREK channel in the C-terminal region, while the opening and closing of the channel are mainly controlled by the selectivity filter. This specific area within the channel determines which ions can pass through. "Only through the interplay of these two regions is it decided whether ions can pass or not. However, the selectivity filter and the C-terminal region of a TREK channel are not adjacent, and how this interplay works through long-range coupling was previously unknown," reports Berke Türkaydin, a PhD student in Han Sun's group and first author of this study. The researchers at FMP analyzed the dynamics of the channels and investigated how the proteins move during phosphorylation on the nanosecond to microsecond timescale. They used large-scale and time-intensive molecular dynamics simulations combined with functional electrophysiology validations. "Our work allowed us to elucidate in detail how these two important functions are coupled and to prove that phosphorylation plays a central role in the opening and closing of TREK channels," says Prof. Han Sun. The researchers demonstrated that the two key functions are linked through two pathways and identified several critical interactions that mediate the coupling. These insights highlight the central importance of protein dynamics in many different biological processes.
The next step involves developing and optimizing new small molecule modulators that can better and more specifically inhibit or activate TREK channels. This work could be significant for the development of therapeutics for diseases in which the function of TREK channels is disrupted.
Thomas Baukrowitz, Johann Biedermann, Elena Barbara Riel, Marcus Schewe, Friederike Schulz, Han Sun, Berke Türkaydin
Atomistic mechanism of coupling between cytosolic sensor domain and selectivity filter in TREK K2P channels






