Key takeaways
● A type of plastic called PEDOT that can conduct electricity is currently used to protect the internal components of electronic devices from static electricity and in organic solar cells and electrochromic devices, but it also has the ability to store electric charge somewhat like a battery.
● UCLA chemists have created a new type of textured, fur-like PEDOT film with more surface area to store charge and built a supercapacitor with it that stored nearly ten times more charge than conventional PEDOT and lasted nearly 100,000 charging cycles.
● The advance could lead to supercapacitors that can meet some energy storage demands as the world transitions to renewable, sustainable energy production.
Plastics have shaped our modern world and changed the way we live. For decades, they have been primarily used in electronics for their excellent insulating properties. But in the 1970s, scientists accidentally discovered that some plastics can also conduct electricity. This finding revolutionized the field and opened the door to applications in electronics and energy storage.
One of the most widely used electroconductive plastics today is called PEDOT, short for poly(3,4-ethylenedioxythiophene). PEDOT is a flexible, transparent film often applied to the surfaces of photographic films and electronic components to protect them from static electricity. It is also found in touch screens, organic solar cells and electrochromic devices, such as smart windows that switch from light to dark at the press of a button. However, PEDOT's potential for energy storage has been limited because commercially available PEDOT materials lack the electrical conductivity and surface area needed to hold large amounts of energy.
UCLA chemists are addressing these challenges with an innovative method to control the morphology of PEDOT to grow nanofibers precisely. These nanofibers exhibit exceptional conductivity and expanded surface area, both of which are crucial for enhancing the energy storage capabilities of PEDOT. This approach, described in a paper published in Advanced Functional Materials, demonstrates the potential of PEDOT nanofibers for supercapacitor applications.
Unlike batteries, which store energy through slow chemical reactions, supercapacitors store and release energy by accumulating electrical charge on their surface. This allows them to charge and discharge extremely quickly, making them ideal for applications requiring rapid bursts of power, such as regenerative braking systems in hybrid and electric vehicles and camera flashes. Better supercapacitors are, therefore, one route to reduced dependence on fossil fuels.
The challenge with supercapacitors, however, is creating materials with enough surface area to hold large amounts of energy. Traditional PEDOT materials fall short in this regard, which limits their performance.
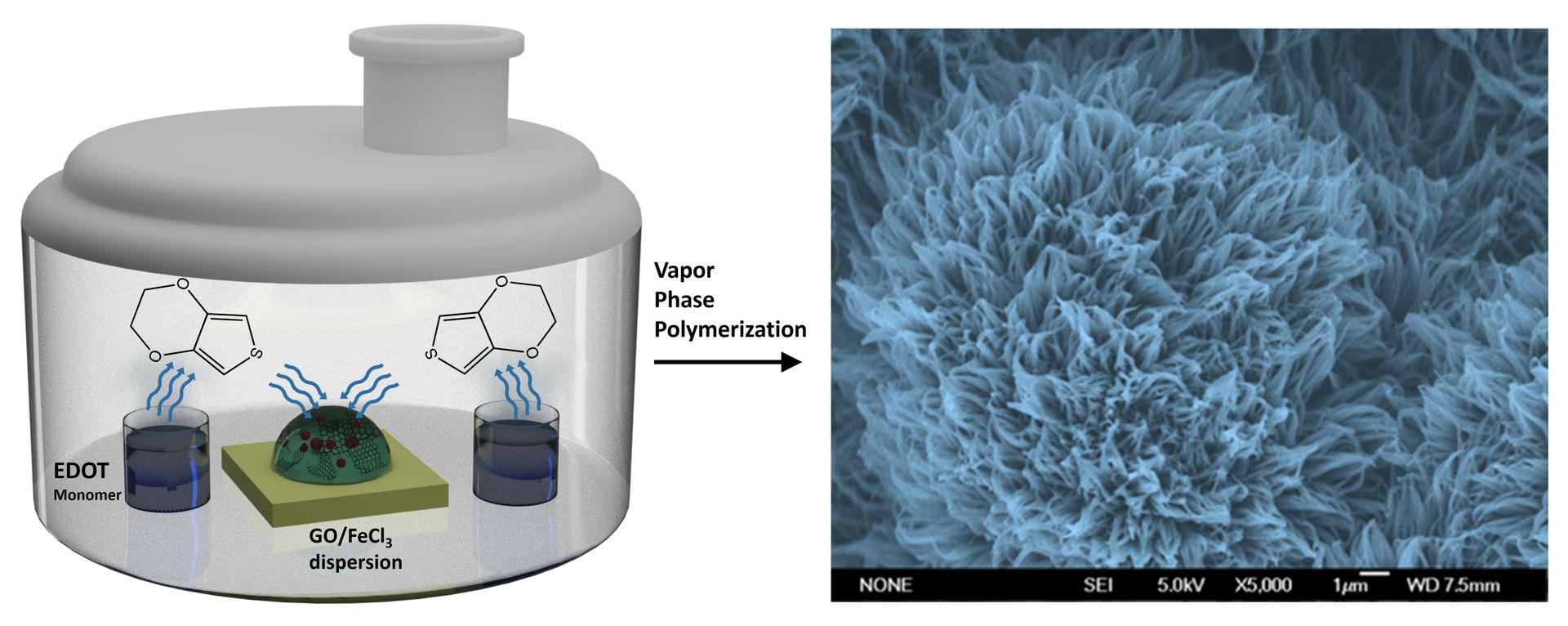
The UCLA chemists produced the new material through a unique vapor-phase growth process to create vertical PEDOT nanofibers. These nanofibers, resembling dense grass growing upward, dramatically increase the material's surface area, allowing it to store more energy. By adding a drop of liquid containing graphene oxide nanoflakes and ferric chloride on a graphite sheet, the researchers exposed this sample to a vapor of the precursor molecules that eventually formed the PEDOT polymer. Instead of developing into a very thin, flat film, the polymer grew into a thick, fur-like structure, significantly increasing the surface area compared to conventional PEDOT materials.
"The material's unique vertical growth allows us to create PEDOT electrodes that store far more energy than traditional PEDOT," said corresponding author and UCLA materials scientist Maher El-Kady. "Electric charge is stored on the surface of the material, and traditional PEDOT films don't have enough surface area to hold very much charge. We increased the surface area of PEDOT and thereby increased its capacity enough to build a supercapacitor."
The authors used these PEDOT structures to fabricate supercapacitors with excellent charge storage capacity and extraordinary cycling stability, reaching nearly 100,000 cycles. The advance could pave the way for more efficient energy storage systems, directly addressing global challenges in renewable energy and sustainability.
"A polymer is essentially a long chain of molecules built out of shorter blocks called monomers," said El-Kady. "Think of it like a necklace made from individual beads strung together. We heat the liquid form of the monomers inside a chamber. As the vapors rise, they react chemically when they come in contact with the surface of the graphene nanoflakes. This reaction causes the monomers to bond and form vertical nanofibers. These nanofibers have (a) much higher surface area, which means they can store much more energy."
The new PEDOT material has shown impressive results, exceeding expectations in several critical areas. Its conductivity is 100 times higher than that of commercial PEDOT products, making it far more efficient for charge storage. What's even more remarkable is that the electrochemically active surface area of these PEDOT nanofibers is four times greater than that of traditional PEDOT. This increased surface area is crucial because it allows for much more energy to be stored in the same volume of material, significantly boosting the performance of supercapacitors.
Thanks to the new process, which grows a thick layer of nanofibers on the graphene sheet, this material now has one of the highest charge storage capacities for PEDOT reported to date — more than 4600 milliFarads per square centimeter, which is nearly one order of magnitude higher than conventional PEDOT. On top of that, the material is incredibly durable, lasting through more than 70,000 charging cycles, far outlasting traditional materials. These advances open the door for supercapacitors that are not only faster and more efficient but also longer-lasting, which are essential qualities for the renewable energy industry.
"The exceptional performance and durability of our electrodes shows great potential for graphene PEDOT's use in supercapacitors that can help our society meet our energy needs," said corresponding author Richard Kaner, a UCLA distinguished professor of chemistry and of materials science and engineering, whose research team has been at the forefront of conducting polymer research for over 37 years. As a doctoral student, Kaner contributed to the discovery of electrically conductive plastic by his advisors Alan MacDiarmid and Alan Heeger, who later received a Nobel Prize for their work.
Other authors on the study include Musibau Francis Jimoh, Gray Carson and Mackenzie Anderson, all of whom are also at UCLA.






