Ultrasensitive detection of NO using a conductive 2D metal-organic framework
Detection of nitric oxide (NO) is important for monitoring air quality because the NO released in the combustion of fossil fuels contributes to acid rain and smog. In medicine, NO is an important messenger molecule and serves as a biomarker for asthma. In the journal Angewandte Chemie, a research team now reports a material that can detect NO reversibly, with low power, and with high sensitivity and selectivity: a copper-containing, electrically conducting, two-dimensional metal-organic framework.
© Wiley-VCH, re-use with credit to 'Angewandte Chemie' and a link to the original article.
Metal-organic frameworks (MOFs) are latticelike structures consisting of metal "nodes" connected by organic bridges (ligands). An emerging class of MOFs are electrically conducting structures consisting of layers. These 2D-cMOFs have demonstrated great potential as chemiresistive sensors that react to the presence of specific molecules with a change to their electrical resistance, which may allow for particularly sensitive and low-power detection of toxic gases. Problems with such systems have included cross-reactivity with a variety of gases and limited reusability due to irreversible binding of the analytes.
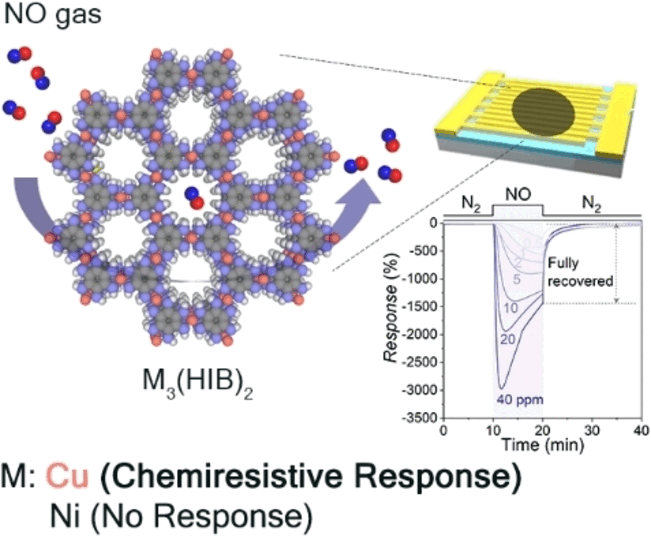
Katherine A. Mirica, Christopher H. Hendon, and their team at Dartmouth College (Hanover, NH, USA), the University of Oregon (Eugene, OR/USA), and Ulsan National Institute of Science and Technology (South Korea), have now developed a reusable 2D-cMOF for the highly selective detection of NO. They chose to use a 2D-cMOF based on copper and hexaiminobenzene, Cu3(HIB)2. Thanks to their different synthetic strategy (the linker was added as an undissolved powder to a solution of Cu2+ ions and potassium acetate), the team produced a material with significantly higher crystallinity (rod-shaped crystallites about 500 nm in length) than has previously been attained.
The crystallites consist of stacked layers of a weblike structure of six-membered rings linked together by copper ions bound to their nitrogen atoms. Spectrometric analyses and computations revealed that the binding sites for NO were Cu-bis(iminobenzosemiquinone) units of the copper-2D-cMOFs. An analogous compound made with nickel instead of copper demonstrated no significant absorption of NO. Evidently, copper ions with a single positive charge, which are present in small amounts in the structure besides those with a twofold positive charge, play an important role in binding NO. Computational studies suggest that the adsorbed NO significantly distorts the structure, destabilizing the bound state, which is the primary cause for the desirable reversibility of the NO adsorption.
This new sensor material detects NO at room temperature and low voltage (0.1 V) with high sensitivity (detection limit about 1.8 ppb) and could be reused for at least seven cycles without regeneration. Quantitative measurements of NO were also successful in the presence of moisture, and showed high enhancement of sensor signal towards NO in comparison to other gases, such as nitrogen dioxide, hydrogen sulfide, sulfur dioxide, ammonia, and carbon monoxide and dioxide.
(3153 characters)
About the Author
Dr. Katherine Mirica is an Associate Professor of Chemistry at Dartmouth College. Her main research direction focuses on the development of multifunctional porous materials capable for sensing, filtration, and detoxification of hazardous chemicals. She is also an Associate Editor of ACS Sensors.
Copy free of charge-we would appreciate a transcript/link of your article. The original articles that our press releases are based on can be found in our online pressroom.






