Researchers at the University of Würzburg have discovered a process that breaks down mRNA molecules in the human body particularly efficiently. This could be useful, for example, in the treatment of cancer.
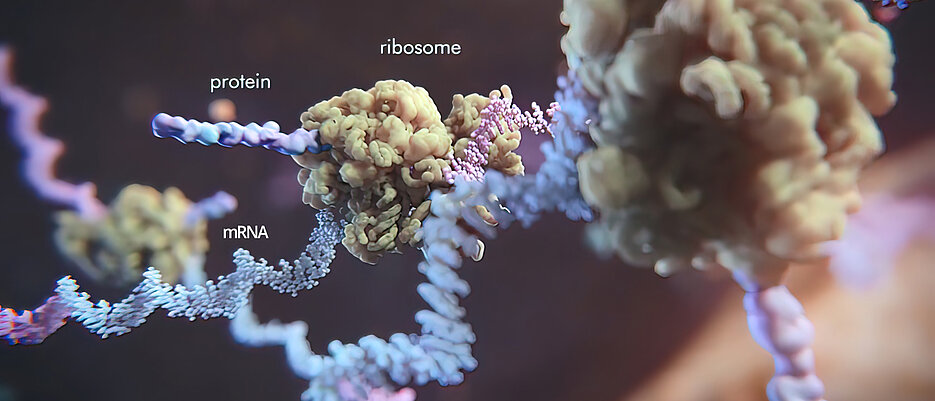
They are like the architects of our body: messenger ribonucleic acids, or mRNA for short. They contain detailed blueprints for proteins, which are read and translated by their "colleagues", the ribosomes. We could not survive without the proteins in our bodies - they control cell division, provide a strong immune system and make our cells resistant to external attack.
As in "real construction", some blueprints at the cellular level require additional instructions - for example, if a protein needs to be produced particularly quickly or if the blueprint is faulty. In our body, this role is played by so-called RNA modifications, small chemical changes that act like additional comments attached to individual components of the mRNA.
New Degradation Process for MRNA Discovered
Researchers at the University of Würzburg (JMU) in Bavaria, Germany, have now focused on a specific modification, N6-methyladenosine (m6A). "m6A is interesting for science because this modification is often altered in people who suffer from metabolic disorders, cancer or heart disease", explains bioinformatician Kathi Zarnack. "Its function: When m6A is attached to an mRNA, it triggers the degradation of the mRNA as soon as the first proteins have been produced according to the blueprint it contains. This is particularly important for proteins, of which too many must not be produced as this would be harmful to the cell." The Würzburg researchers were the first to discover and observe this degradation process: It couples the degradation of an mRNA directly to the proteins produced and is significantly faster and more efficient than previously known mechanisms for mRNA degradation.
Crucially, this particular pathway only works when m6A is present in specific regions of the mRNA. In this way, m6A particularly "comments" on the blueprints for proteins involved in cell differentiation - that is, whether a cell will exist as a nerve cell, muscle cell, skin cell or some other form.
Drugs that control the addition of m6A to mRNA could take advantage of this process. By specifically suppressing m6A, it would be possible to produce more proteins with desirable functions - and, conversely, to inhibit the production of undesirable proteins. The problem: Until now, it has been difficult for scientists to predict the effects of such drugs because it was not known in which regions of the mRNA the m6A modification had to be located in order to trigger degradation. "With our study, we are now contributing to a better understanding and more precise prediction of which mRNAs are particularly sensitive to these drugs", says biochemist and RNA biologist Julian König, Zarnack's colleague.
Next Research Steps
In the future, the researchers plan to investigate in more detail how m6A-marked mRNA is degraded, for example, how ribosomes recognise the modification, and how targeted mRNA degradation by m6A can be used clinically.
In addition to the Würzburg researchers, the Institute of Molecular Biology (IMB) in Mainz and the Goethe University in Frankfurt are also involved in the study, which is funded by the German Research Foundation as part of the Collaborative Research Centre TRR 319 "RMaP: RNA Modification and Processing".
About the Study
m6A sites in the coding region trigger translation-dependent mRNA decay. You Zhou, Miona Ćorović, Peter Hoch-Kraft, Nathalie Meiser, Mikhail Mesitov, Nadine Körtel, Hannah Back, Isabel S. Naarmann-de Vries, Kritika Katti, Aleš Obrdlík, Anke Busch, Christoph Dieterich, Štěpánka Vaňáčová, Martin Hengesbach, Kathi Zarnack, Julian König. Molecular Cell.21 November 2024. DOI: 10.1016/j.molcel.2024.10.033






