© 2023 Alain Herzog
EPFL spin-off Artiria has developed a wire with a guidable tip that makes it easier for neurosurgeons to navigate the dense network of cerebral arteries and get to the source of strokes. Its system has just received clearance from the US Food and Drug Administration (FDA).
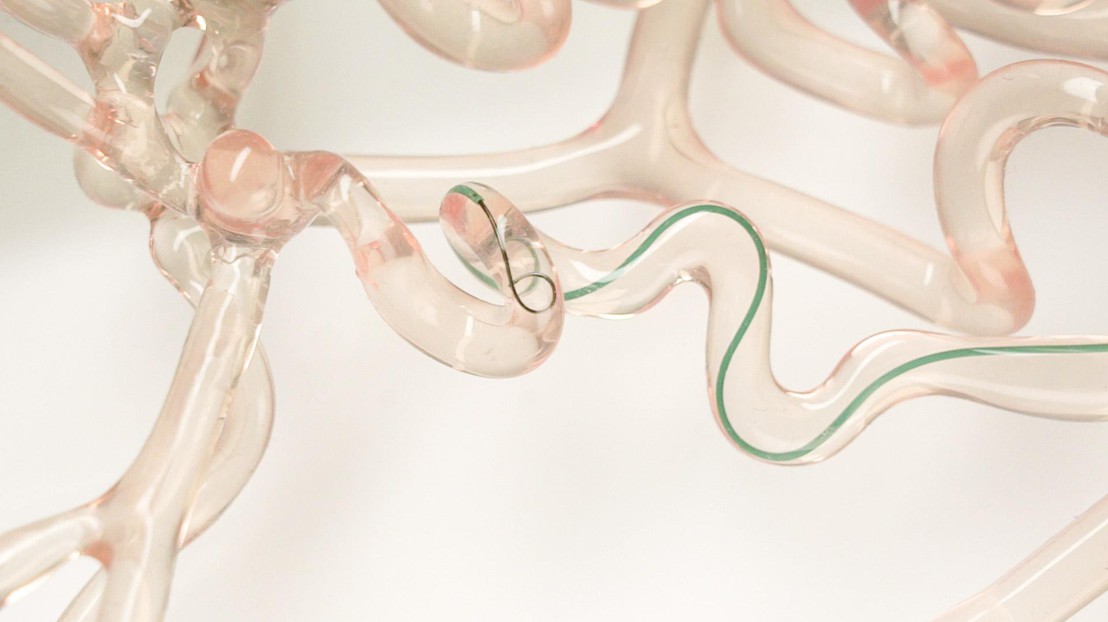
Our brains contain an intricate network of arteries that carry blood throughout the organ along winding paths. For neurosurgeons, following these paths with a wire - which is just a third of a millimeter in diameter and enters the body through the femoral artery - to reach an obstructed blood vessel can be tricky. For instance, if they want to point the wire in a different direction, they often have to pull the instrument out and then reinsert it, lengthening surgery times and increasing the risk of complications. But the new wire developed by Artiria is set to change all that. Its tip can be controlled by pressing a button on its handle, through an apparatus that runs entirely on mechanical forces. Artiria just received FDA clearance to test and market its system in the US.
The figures on strokes are startling. According to the World Health Organization, strokes are the leading cause of disability and the second-leading cause of death worldwide. One-fourth of people over 25 can expect to experience one during their lifetime. And when a stroke occurs, time is of the essence - rapid treatment can improve a patient's prognosis considerably. "While strokes can be caused by a ruptured aneurysm, 80% of the time they're due to a blood clot in the brain," says Guillaume Petit-Pierre, Artiria co-founder and CEO. In combination with drug treatments to dissolve the clot, the surgical act, facilitated by the real-time visualization of the instruments by x-rays, makes it possible to extract the clot mechanically. The wire serves as a guide so that the other instruments needed for the operation can be inserted. Before creating their company, Petit-Pierre and Marc Boers - the other Artiria co-founder - spoke with several neurosurgeons and watched them operate several times in order to gain a thorough understanding of the techniques they use. The founders' goal was to develop a device that would fit in seamlessly with existing procedures. "We were able to get the FDA clearance so quickly because our wire is similar to existing ones in so many respects," says Petit-Pierre.
These micro-cuts, just a few tens of microns in size, are made from a superplastic alloy, ensuring the necessary flexibility of the wire tip while avoiding injury to the arterioles of the brain.
Useful for other types of post-stroke surgeries, too
Petit-Pierre and Boers tested their system on 3D-printed, clear-silicone models of cerebral arteries, and found that it didn't create any major differences for neurosurgeons. It simply has an extra button on the handle that neurosurgeons can press when they want the tip to bend. A tiny pull wire relays the (slight) mechanical force created from pressing the button all along the structure of the instrument all the way to its 2-centimeter-long deflectable tip. The tip is reinforced on the side connected to the pull wire, and the other side is designed to follow the movement easily. The system may appear simple to the human eye, but fabricating its microscopic-scale parts was a considerable feat of engineering. "These micro-cuts, just a few tens of microns in size, are made from a superplastic alloy, ensuring the necessary flexibility of the wire tip while avoiding injury to the arterioles of the brain. The technological feat also consists in integrating a radio-opaque element into an extremely small volume, enabling the tip of the tool to be visualized during x-ray navigation", explains Guillaume Petit-Pierre. In order to guarantee flawless product cleanliness, the first versions of this system were assembled in EPFL's clean room.

The two founders are also exploring other applications for the underlying technology, which came out of EPFL's Microsystems Laboratory 4 (LMIS4). "For example, we worked with the Wyss Center in Geneva to see if our wire could be used to lower spasms observed during hemorrhaging storkes," says Petit-Pierre. Here, the wire could be used to target a specific artery using flexible thin-film electrodes. "There's currently no effective way to treat cerebral vasospasms, even though they're known to be a leading cause of disability and death after aneurysm-triggered strokes."
Petit-Pierre and Boers are old friends and decided to create a startup around ten years ago, while on a backcountry skiing trip. At the time, Petit-Pierre worked in the medtech industry and Boers was already involved in other startups. Petit-Pierre did his PhD at LMIS4 - headed by Philippe Renaud, who was recently named professor emeritus - and the atmosphere there convinced him to try his hand at entrepreneurship. Some 25 businesses have spun off from Prof. Renaud's lab, so there were plenty of role models to learn from. The core elements of Artiria's system came from Petit-Pierre's PhD thesis at LMIS4. With Boers he filed a patent application and created the company in 2019.
Artiria was awarded 2.7 million francs in funding under the European Innovation Council Accelerator Program - although the financing actually came from the Swiss government (SEFRI) since Switzerland no longer has a framework agreement with the EU - and has raised 4.1 million francs from investors. The medtech firm is ranked among Switzerland's top 100 startups. The two founders plan to launch a more substantial funding round in the coming months, the proceeds of which will be used to expand its seven-person team and validate the product's clinical use.






