On the wall of Dian Yang's office hangs a painting of a "Pandora's box of cancer" being opened with a molecular key. From inside the box, strands of DNA emerge, transform into cancer cells, morph into neuron-like protrusions, and then shape-shift into bats that fly off into a dark night.

The painting, which Yang created himself, is a metaphor for Yang's work exploring how cancers gain the ability to spread through the body.
As an early-career scientist, Yang has already made inroads into discovering molecular changes that make lung cancers more likely to metastasize. He has also helped develop and fine-tune a molecular recording technology, based on CRISPR, that lets him follow the evolution of cancer cells on a cell-by-cell basis. That lets him pinpoint exactly when and how a tumor transforms and spreads.
"The hope is that each time we can discover one of these molecular keys that opens the Pandora's box of tumor evolution, we might be able to develop a way to stop it," says Yang, who joined Columbia University's Vagelos College of Physicians and Surgeons in late 2023 as an assistant professor of molecular pharmacology and therapeutics.
This year, Yang received a $1.5 million NIH High-Risk, High-Reward Research grant to continue his research mapping how and why lung cancers spread. He says that support, coupled with the collaborative environment at Columbia, will help his research advance.
The deadliest cancer
Growing up in China and fascinated by the natural world, Yang was a frequent visitor to the Beijing Zoo and China Science and Technology Museum during his childhood. As an undergraduate at Cornell University, that love of science began extending to biochemistry and cancer research when he worked in a lab studying DNA replication.

When he began his PhD at Stanford, Yang rotated through labs that focused on stem cells, regenerative medicine, and cancer.
"I had pretty broad interests across biology," Yang recalls. "But when I rotated in the labs of Monte Winslow and Julien Sage and learned just how deadly lung cancer is, I felt like it was a really important medical problem to tackle."
In the U.S., lung cancer is the second most common cancer in both men and women, and by far the leading cause of cancer deaths, accounting for about one in five of all cancer deaths. Symptoms of lung cancer don't usually appear until the cancer has already begun spreading to other parts of the body; only 16% of lung cancers are diagnosed while still confined to the lungs, when treatments are more likely to be effective.
At Stanford, Yang began studying what makes small cell lung cancer cells gain the ability to travel to other parts of the body. He identified a protein, NFIB, that can promote metastasis. When lung cancer cells gain extra NFIB-something that can happen as a tumor evolves over time-the structure of their DNA changes, letting the cells activate gene programs usually only turned on in migrating brain cells during embryo development.
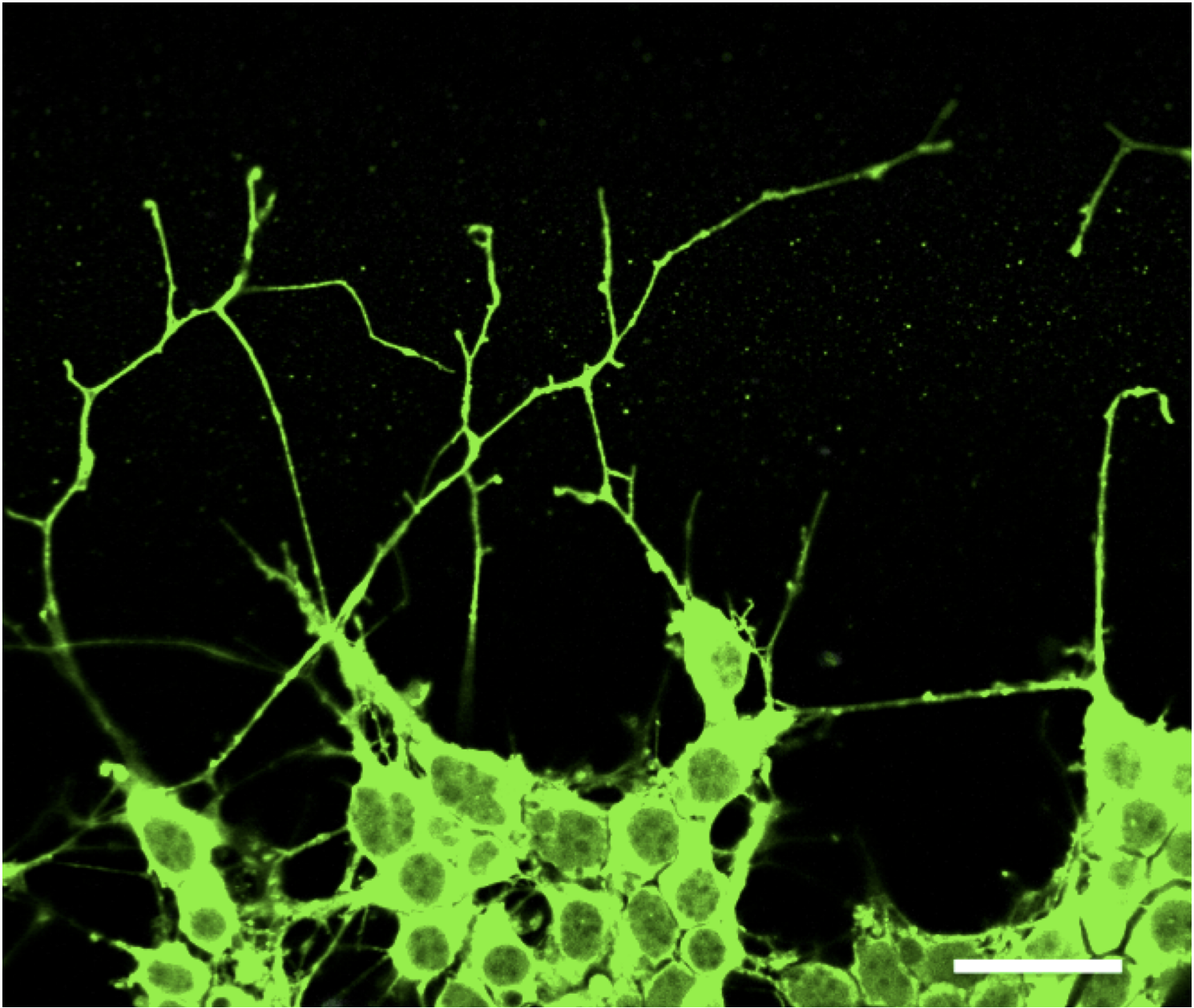
As lung cancer cells evolve, Yang has found that some start extending long structures that look like the axons of neurons and start to migrate. Image provided by Dian Yang/Columbia University Vagelos College of Physicians and Surgeons.
"It was really striking," Yang says. "These lung cancer cells start extending long structures that look like the axons of neurons and then start to migrate."
Cells with high levels of NFIB, Yang and his colleagues showed, were more likely to extend those structures and help them spread to the lymph node and liver-sites of metastasis most seen in people with lung cancer. That finding, published Cell in 2016, is what inspired the art on Yang's wall.
Help from CRISPR
When he completed his PhD work, Yang wanted to keep tackling lung cancer metastasis and was intrigued by how new, emerging technologies-including approaches that analyze cells one-by-one-could help advance his research. He joined the lab of Jonathan Weissman at the Massachusetts Institute of Technology, where researchers were developing these kinds of experimental tools.
"With single-cell sequencing technologies, we could capture the heterogeneity of tumors and start to ask questions about what makes just some cells in a tumor, but not all, gain the ability to metastasize," explains Yang.
At MIT, Yang developed a new method based on CRISPR technology to track how the individual cells within a tumor evolved. By inducing cancer-causing mutations in normal lung cells, as well as initiating an evolving genetic barcode that labeled each cell and its progeny, Yang could build a detailed family tree of every cell in a large tumor. "This suddenly gave us unprecedented resolution to understand how you go from one cancer cell all the way to this diverse, aggressive collection of tumor cells."
"This suddenly gave us unprecedented resolution to understand how you go from one cancer cell all the way to this diverse, aggressive collection of tumor cells," says Yang. "You can see how some cancer cells evolve differently from others and branch out over time."
When Yang and his colleagues started analyzing these family trees of cancer cells, he found that cells on expanding branches of the tree have unique gene expression patterns that correlate with poor patient survival-and metastatic cells mostly originate from these specific branches of the tumor family tree.
Surprisingly, they also found that changes in DNA sequences, passed from cell to cell, were not the major contributor of tumor metastasis. Instead, epigenetic changes, which do not alter the DNA sequence but can change gene expression in cells, seemed critical.
In his new lab at Columbia, Yang is trying to track the origins and dynamics of metastasis development in lung cancers and understand the molecular details that lead to metastasis. These new insights could eventually lead to new ways of predicting-or stopping-the spread of cancer. In addition, he also wants to study how the non-cancerous cells around a tumor, like immune cells, play a role in this process.
"I'm still driven by a natural curiosity about the world," says Yang. "I am always excited about doing new, cool stuff and answering questions that could not be addressed before."






