Researchers in the UC Davis College of Biological Sciences have received a grant to study the role of the cerebellum in autism.
"We need a more holistic understanding of the brain circuits that drive autism," said Alex Nord, an associate professor of neurobiology, physiology and behavior, and a researcher at the Center for Neuroscience and the UC Davis MIND Institute. "The cerebellum is a key component that has been largely overlooked until recently."
Nord partnered with Diasynou Fioravante, also an associate professor of neurobiology, physiology and behavior and Center for Neuroscience researcher. They received an R21 grant from the National Institute of Mental Health (NIMH). Announced in November, it will provide $435,000 of funding over the next two years.
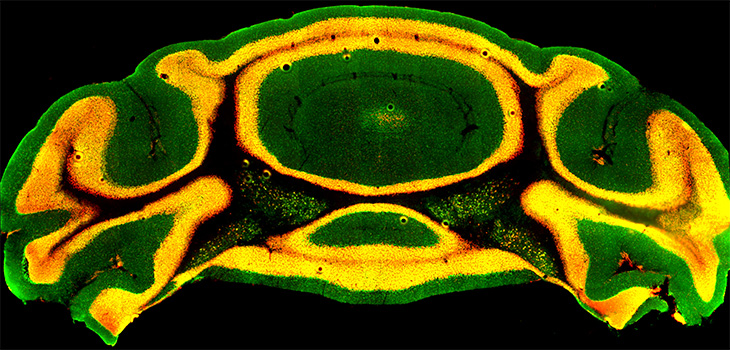
The grant will allow them to study how a potent autism-associated gene, called chromodomain helicase DNA binding protein 8 (Chd8), alters function in the cerebellum, the portion of the brain that plays a crucial role in physical movement. They'll research how this drives autism-like behaviors. Their work ties together two emerging trends in autism research.
Surprising discoveries in autism
Researchers long believed that autism involved genes that regulate communications between neurons — especially the synapses, where neurotransmitters carry signals from one neuron to another. But starting in the early 2010s, genetic studies revealed that many autism-associated genes actually play a very different role: They encode proteins that reside far from the synapses — in the cell's nucleus, where the DNA is located, and regulate the activity of hundreds of other genes.
"This was a huge surprise," Nord said. Chd8, a typical member of this group, regulates how tightly DNA is packaged and coiled with proteins. As such, it may regulate many genes that govern the formation and function of synapses.
At the same time, another major area of study is advancing the field of autism research. Scientists had assumed that autism was driven by changes in the cerebral cortex, which performs all sorts of tasks. These include recognizing words and faces, and "executive function" — controlling working memory, guiding our spotlight of attention, and making choices — such as whether to act on the impulse to withdraw one's hand when an unfamiliar dog approaches.
But in 2012, researchers reported that mice with mild abnormalities in the cerebellum developed behaviors that resembled autism in humans, such as reduced social interaction with other mice. "The autism field was really rocked by this discovery," Fioravante said.
People had previously believed that the cerebellum mainly coordinates body movements such as walking, speaking, and typing. "When you damage the cerebellum, it causes profound movement problems, and this probably made it difficult to see more subtle changes in behavior," Fioravante explained.
But she points out that the cerebellum has significant connections with brain structures like the prefrontal cortex, which guides executive function, and limbic system, which regulates sociability, mood and emotions. People with cerebellar injuries often show autism-like changes in their emotions and social interactions.
Then in 2017, Nord and his colleagues reported a discovery that tied together these two intriguing threads: They found that the autism risk gene Chd8 helped guide the development of the cerebellum. Mice with one non-functioning copy of the gene had smaller cerebella.
That discovery "planted the idea of our project," Fioravante said. She and Nord sat in offices next door to one another. And while Nord studied Chd8, she worked on the cerebellum. In 2021 they applied for, and received, an earlier NIMH grant, to study how Chd8 influences the development of the cerebellum in mice, before and shortly after birth. Fioravante also received a pilot grant from the Behavioral Health Center of Excellence at UC Davis that launched Chd8 cerebellar studies in adult mice.
Working on these earlier grants from 2021 to 2023, they found that Chd8 mutations in mice triggered changes in the cerebellum and in behavior that resemble what is seen in autistic humans. For example, mice with a mutant copy of the gene had impaired social cognition. While regular mice prefer to explore and interact with mice they have not met before, mice with mutant Chd8 had more restricted interests — preferring mice or objects that they already knew.
We need a more holistic understanding of the brain circuits that drive autism. The cerebellum is a key component that has been largely overlooked until recently."-Alex Nord, associate professor of neurobiology, physiology and behavior
New targets for treatment
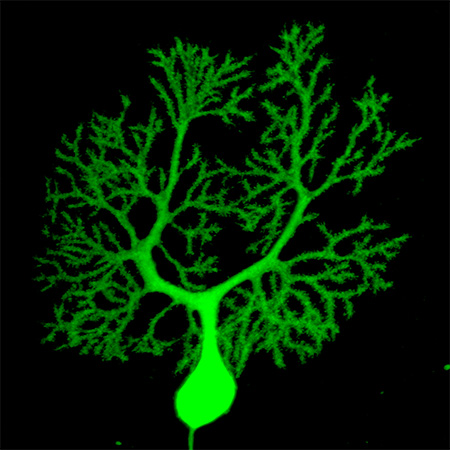
With the new grant, Fioravante and Nord will pick up where they left off. In adult mice with normally developed cerebella, they will use genetic tools to disrupt Chd8. They will examine how loss of Chd8 alters gene expression and function in neurons of the cerebellum. They'll also study how the connections between it and other brain areas change. They will also research whether disruption of Chd8 causes autism-like behavioral changes, such as reduced social interaction with other mice, or reduced interest in novelty. Cesar Canales, an assistant professional researcher who has expertise in cerebellar anatomy, will take part in these studies.
These experiments "will help shed light on the totality of what the cerebellum does," Fioravante said — getting beyond the traditional narrow view that it mainly coordinates movement. The team also hopes to uncover new autism intervention strategies.
Although autism involves early brain development, the condition usually isn't diagnosed until children are years older — when the abnormal brain connections are already established, potentially making intervention difficult.
In these upcoming studies, Nord and Fioravante hope to explore whether the treatment window for autism can be extended. "If we see changes in behavior or neural circuits when Chd8 is disrupted in the developed cerebellum, then we know there is a treatment target," Nord said.
These studies could also shed light on schizophrenia and obsessive-compulsive disorder, which occur more frequently in people with mutations in this gene, said Nord. "It's a molecular handle, an entry point into the pathophysiology of these other complex diseases."







