Researchers at Nano Life Science Institute (WPI-NanoLSI), Kanazawa University report in Nano Letters how the flexibility of a protein hinge plays a crucial role in the transfer of proteins in key cell processes.
————————————————————————————————————————————————————————————————
Ubiquitination - the addition of the protein ubiquitin – is a key stage in many cell processes, such as protein degradation, DNA repairs, and signal transduction. Using high-speed atomic force microscopy (HS-AFM) and molecular modelling, researchers led by Hiroki Konno and Holger Flechsig at WPI-NanoLSI, Kanazawa University have identified how the mobility of a ubiquitination related enzyme hinge allows ubiquitination to take place.
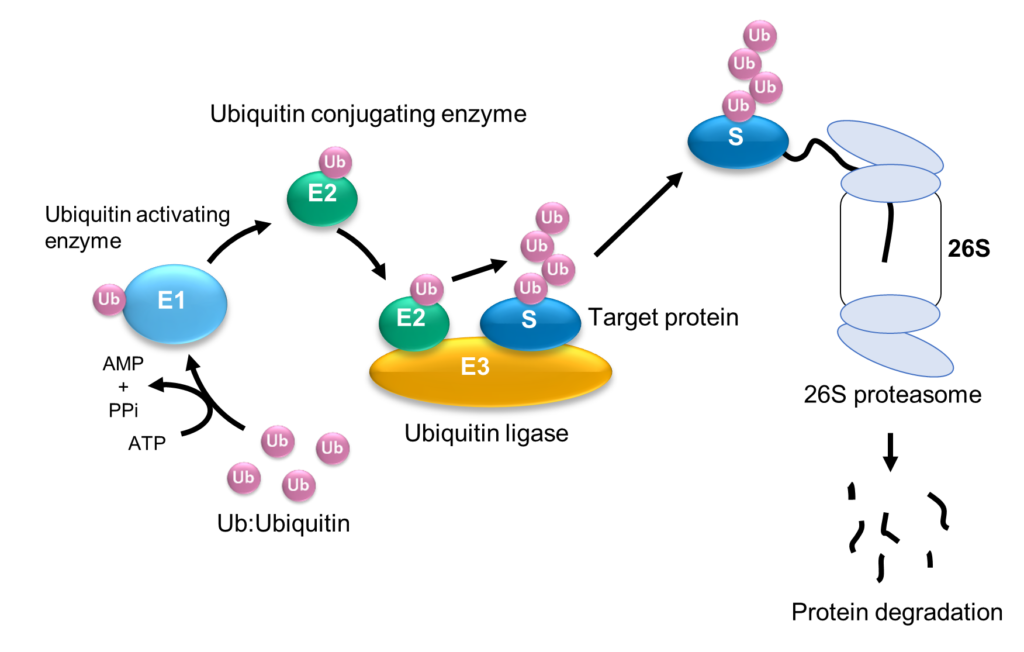
Previous studies have identified a number of enzymes that facilitate ubiquitination, including an enzyme that activates ubiquitin (E1), an enzyme that conjugates it (E2), and an enzyme that catalyzes ubiquitin protein joining (i.e. a ligase, E3) to target protein (Fig. 1). The HECT-type E3 ligase is characterized by a HECT domain that comprises an N lobe with the E2-binding site and a C lobe with a catalytic Cys residue, A flexible hinge connects the two lobes, leading to the hypothesis that ubiquitination is facilitated by the rearrangement of the protein around this hinge. Konno and their collaborators deployed their high-speed atomic force microscope to hunt for evidence that this was the case.
The researchers noted that when the HECT domain was crystallized with a type of E2 enzyme, it formed an L shape such that the distance between the catalytic Cys residue of the HECT domain and the catalytic Cys of the E2 enzyme was 41 Å - too far for the transfer of ubiquitin. However, in its catalytic conformation the HECT domain has a different shape where the distance between the two catalytic Cys residues is much closer - just 8 Å - so this is thought to be a "catalytic conformation".
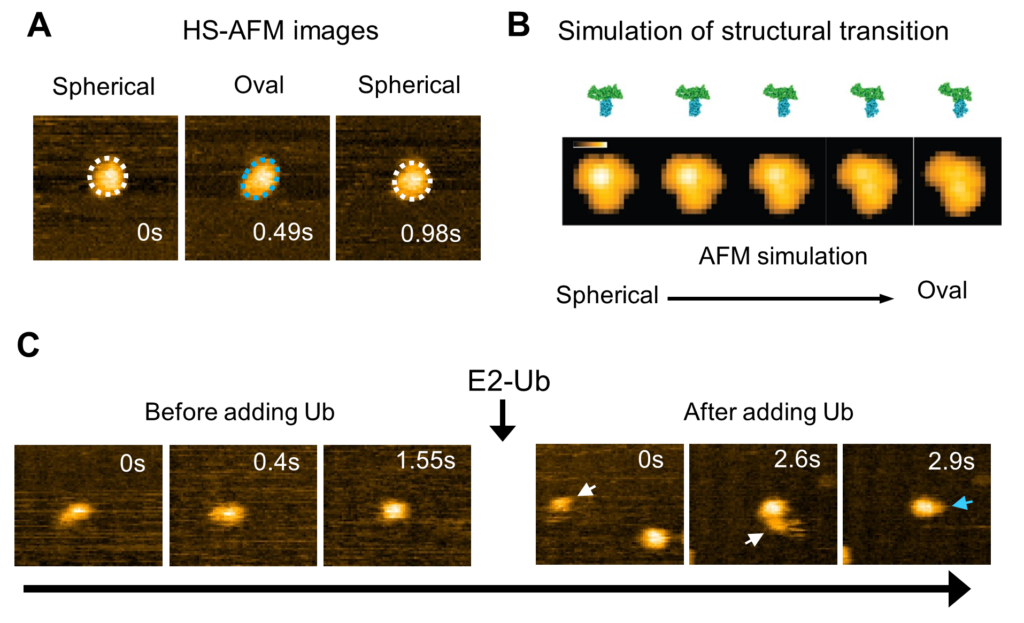
Analysis of HS-AFM images of a wild-type HECT domain of E6AP revealed two conformations - one of which looked spherical and the other oval (Fig. 2). Using AFM simulations they attributed the oval shapes to the L conformation and spherical shapes are either the catalytic conformation or the so called inverted T conformation, which had been observed in the another type of HECT domain where the distance between the Cys residues is 16 Å (Fig. 2). To overcome the spatio-temporal resolution limitations of imaging, the experiments were complemented by molecular modelling to visualize HECT domain conformational motions at the atomistic level. Simulation AFM was used to generate a corresponding pseudo AFM movie, which clearly showed the change from spherical to the oval shaped topography (Fig. 2).
"Although experimental limitations do not allow us to resolve the intermediate conformations," explain the researchers in their report of the work. "The performed modeling provides evidence that the transitions between spherical and oval HECT domain shapes observed under HS-AFM correspond to functional conformational motions under which the C-lobe rotates relative to the N-lobe, thereby allowing the change between catalytic and L-shape HECT conformations."
Further experiments with mutant HECT domains with less flexibility in the hinge revealed no flipping between conformations - the mutant HECT domains were locked in the catalytic conformation. They also found that while these mutant HECT domains could form two ubiquitin proteins joined together more efficiently than the wild type.
E6AP, the HECT-type E3 in this study, interacts with E6 protein derived from human papillomavirus (HPV) and ubiquitinates p53, a tumor suppressor protein. It is also known that ubiquitination of p53 by E6AP and E6 is a major cause of cervical cancer. However, the mechanism of p53 ubiquitination by the interaction of E6AP and E6 proteins remains unclear. In the future, we will elucidate the structural dynamics of E6AP/E6, and the E6AP/E6/p53 complex with HS-AFM, and clarify how E6 increases the activity of p53 ubiquitination by E6AP.
Fig 1: Ubiquitination and degradation of protein by the ubiquitin-proteasome system. © 2023 Takeda, et al., Published by American Chemical Society
Fig 2: High-speed AFM observation and simulation of the structural dynamics of the HECT domain of E6AP.
(A) HS-AFM image of the HECT domain. Spherical (white dotted line) and oval (blue dotted line) conformational states were observed. (B) Simulated transition process of the structural state of the HECT domain and its pseudo AFM image. (C) Ubiquitin (Ub) is transferred from E2 to E3 (HECT domain). After the addition of ubiquitin-containing E2 (E2-Ub), E2-Ub (white arrow) binds to the HECT domain, and after E2 dissociates, a small particle (blue arrow) that appears to be ubiquitin is added to the HECT domain. © 2023 Takeda, et al., Published by American Chemical Society
Glossary
Ubiquitin
The protein takes its name from the term "ubiquitous" because it is found in almost all tissues. It can be added as a single protein or further proteins added into a chain. This "ubiquitination" is an essential step in many cell processes.
High-speed atomic force microscopy
This imaging technique uses a nanosized tip at the end of a cantilever that is scanned over a sample. It can be used to determine the topography of a sample surface from the change in the strength of forces between the tip and the sample with distance, and the resulting deflection of the cantilever. It was first developed in the 1980s but a number of modifications have augmented the functionality of the technique since. It is better suited to imaging biological samples than the scanning tunnelling microscope developed that had been developed because it does not require a conducting sample.
In the 2000s Toshio Ando at Kanazawa University was able to improve the scanning speed to such an extent that moving images could be captured. This allowed people to use the technique to visualize molecular processes for the first time.
Reference
Authors: Kazusa Takeda†, Holger Flechsig†, Ikumi Muro†, Romain Amyot, Fuminori Kobayashi, Noriyuki Kodera, Toshio Ando and Hiroki Konno
Title: Structural Dynamics of E6AP E3 Ligase HECT Domain and Involvement of a Flexible Hinge Loop in the Ubiquitin Chain Synthesis Mechanism
Journal: Nano Letters
Published on Dec. 6, 2023.
†These authors contributed equally to this work
DOI:10.1021/acs.nanolett.3c04150
URL: https://doi.org/10.1021/acs.nanolett.3c04150