Acting out dreams may seem harmless enough, but it is a little-known early warning sign of the neurodegenerative condition Parkinson's disease.
Parkinson's causes symptoms such as tremors in the hands, slow movement, poor balance and coordination and speech problems.
More than 150,000 Australians are currently living with the disease, which is most common in people over 60.
About 15 per cent of cases are hereditary, and the rest may be associated with environmental factors such as exposure to pesticides or heavy metals. Age is a key risk factor, and men are twice as likely to be diagnosed as women.
While Parkinson's is not considered a fatal condition, it seriously affects patients' quality of life, and increases the risk of life-threatening falls and infections.
Research has identified that inflammation in the brain is a key driver of the disease in the phase before the debilitating motor symptoms begin to appear.
This is known as the prodromal period, and Macquarie University Professor of Cognitive Neurology Simon Lewis says that treatment at this point might be the best opportunity to defeat Parkinson's.
He is currently leading a world-first clinical trial of a drug developed by Sydney biotech company Syntara. Originally intended as a treatment for fatty liver disease and eye disease, it was found to be effective in reducing inflammation in rats' brains.
The study, supported by a grant from Parkinson's UK, is being led by Macquarie University with Oxford University as the second trial site.
Wild dreams are a warning sign
Professor Lewis says on the surface, acting out dreams might seem to be innocuous, but it can be a sign of rapid eye movement (REM) sleep behaviour disorder.

Early intervention: Professor of Cognitive Neurology Simon Lewis, pictured above, is hoping to successfully treat the neuroinflammation that drives Parkinson's disease with a new drug being tested in a world-first trial.
"During dream sleep humans have evolved a system in the brain that stops us from moving and acting out our dreams," he says.
"For our ancestors, this represented an important means of self-protection from predators that hunted at night. However, it appears that damage to the brain's neural pathways in the earliest stages of Parkinson's disease causes a breakdown in this mechanism.
"Over a 12-year period, about 75 per cent of people with isolated REM sleep behaviour disorder go on to develop either Parkinson's or a related condition called dementia with Lewy bodies.
"Once the motor symptoms of Parkinson's appear, like tremors and trouble walking, there has already been substantial loss of more than 50 per cent of the brain's dopamine producing cells.
"If we can successfully treat the neuroinflammation that is driving the disease at the prodromal stage, we could have the chance to 'cure' these conditions before people start losing those brain cells."
For the trial, Professor Lewis is looking for participants aged between 50 and 80 who are regularly having violent dreams where they are punching, kicking or screaming, but not sleepwalking.
They should not have other factors at play such as post-traumatic stress disorder or significant drug or alcohol use.
Other symptoms of prodromal Parkinson's include loss of sense of smell, issues with balance and coordination, difficulty distinguishing between different shades of the same colour, a slight shake in the hands, and handwriting that has become smaller.
"Like the script to a movie"
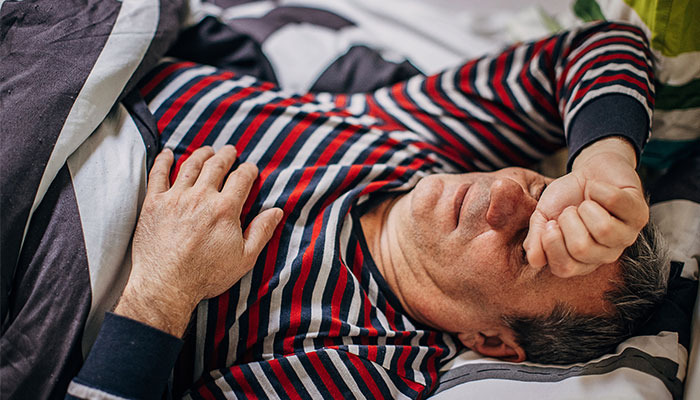
Former high school teacher John Clowes, 73, has just joined the trial. Both his grandfather and half-brother had Parkinson's, but at the time he did not realise the disease might be hereditary.

Violent dreams: John Clowes, pictured above, has joined a clinical trial to test a new drug for Parkinson's after being diagnosed with REM sleep behaviour disorder.
The first indication that something was changing was a shift in his dreams about three years ago.
"I was living out my dreams for the first time, and they were very combative," he says. "I was fighting people, and it was almost like the script to a movie.
"I had never remembered my dreams before, but now I could remember them.
"My wife was noticing too, because I was thrashing around in bed and waking her up.
"It might happen once or twice a week and then not again for months."
After learning this could be an early sign of Parkinson's, and that there was a drug trial available, Mr Clowes decided to volunteer.
He was diagnosed with REM sleep behaviour disorder shortly afterwards, and has just had his first dose of the trial drug.
"If there's a chance that this could work, and it could help other people, that would be excellent," he says.
How to volunteer
Anyone interested in volunteering for the clinical trial will be assessed for symptoms of prodromal Parkinson's disease.
To meet criteria, they need to have undergone a sleep study confirming that they have REM sleep behaviour disorder. Participants will be asked to have a specialised PET scan only available in Melbourne, with travel fully funded.
The study is a randomised control trial, with three-quarters of the people who take part receiving the active drug over six months, while the remaining participants receive a placebo.
Following the treatment phase, all participants undergo follow-up testing, including a second PET scan.
To register your interest, please contact the clinical trial coordinator by email at [email protected]
Simon Lewis is a Professor of Cognitive Neurology at Macquarie Medical School, Macquarie University.






