UNIGE scientists have identified a new type of molecular sensor that enables the malaria parasite to infect human cells or mosquitoes at just the right moment.
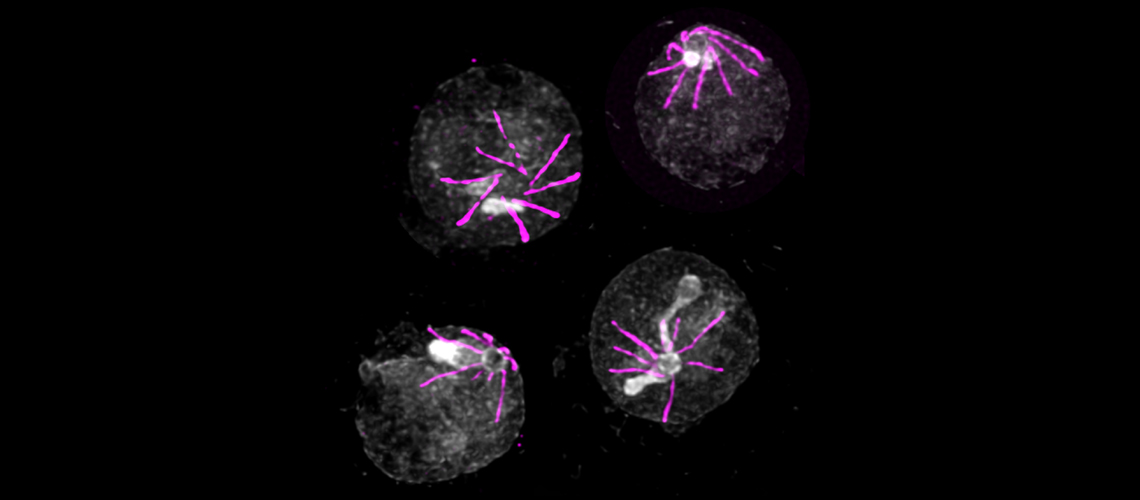
Form of Plasmodium responsible for the destruction of red blood cells as seen by expansion microscopy.
© Vincent Louvel et Eloïse Bertiaux
With almost 250 million cases a year, 621,000 of them fatal, malaria remains a major public health problem, particularly in sub-Saharan Africa. Malaria is a parasitic disease transmitted by mosquitoes and caused by a microbe of the genus Plasmodium. On its journey from mosquito to human, Plasmodium must adapt to the specificities of the many organs and cells it parasitizes. Microbes do not have sensory organs; instead, they have sensors made of proteins to detect molecules specific to the environments they colonize. While most living organisms share the same types of sensors, Plasmodium is an exception. Biologists at the University of Geneva (UNIGE) have identified a new type of sensor that enables Plasmodium to know precisely where it is and what to do. This work, published in the journal Science Advances, opens up the possibility of scrambling the signals perceived by this sensor to disorientate the parasite and thus prevent its replication and transmission.
When a human is bitten by a Plasmodium-infected mosquito, the parasite enters the bloodstream and travels to the liver, where it thrives for around ten days without causing any symptoms. After this period, Plasmodium re-enters the bloodstream, where it parasitizes red blood cells. Once inside the red blood cells, the parasites multiply in a synchronized 48-hour cycle. At the end of each multiplication cycle, the newly-formed parasites leave their host red blood cells, destroying them and infecting new ones. It is this destruction of red blood cells that causes the waves of fever associated with malaria. Severe forms of malaria are linked to the obstruction of blood vessels by infected red blood cells.
When a mosquito bites a human whose blood is infected with Plasmodium, the parasite changes its development program to colonize the intestine of its new host. After a further period of multiplication, Plasmodium returns to the mosquito's salivary glands, ready to infect a new human.
Unknown communication channels
From the warmth of the red blood cell to the depths of the mosquito's intestine via the liver, how does Plasmodium perceive changes in its environment in order to change its development program? "Understanding this very specific biological mechanism is an important step towards countering the parasite," explains Mathieu Brochet, Associate Professor in the Department of Microbiology and Molecular Medicine at the UNIGE Faculty of Medicine, who led this project. "At each stage of its life cycle, the parasite must logically pick up signals that enable it to react correctly, but which ones and how?"
There are small molecules absent in the blood but present in the mosquito that the parasite is able to detect. "Starting from this single known element, we have identified a sensor that enables the parasite to detect the presence of these molecules when it is ingested by a mosquito", explain Ronja Kühnel and Emma Ganga, PhD students in Mathieu Brochet's laboratory and first authors of this study. "This sensor is made up of five proteins. In its absence, the parasite does not realize that it has left the bloodstream for the mosquito, and is therefore unable to continue its development".
Surprisingly, this sensor is also present at other stages of the parasite lifecycle, notably when the parasite has to leave the red blood cell. "We then observe exactly the same mechanism: without this sensor, Plasmodium is trapped in the red blood cells, unable to continue its infection cycle." However, scientists have not identified the human molecules detected by the parasite; identifying them could provide a better understanding of how waves of fever are caused by Plasmodium.
Other parasites also involved
The protein complex discovered here is absent in humans, but is found in the entire family of apicomplexan parasites to which Plasmodium belongs, as well as Toxoplasma, the agent of toxoplasmosis. By identifying this sensor, scientists can now imagine how to scramble the signals perceived by the parasite at different stages of its development, thus disorienting it and blocking its multiplication and transmission.