Mutations in genes encoding mitochondrial aminoacyl-tRNA synthetases are linked to diverse diseases. However, the precise mechanisms by which these mutations affect mitochondrial function and disease development are not fully understood.
The joint team led by Dr. GE Wanzhong from the Women's Hospital, Zhejiang University School of Medicine and Prof. GUAN Min-Xin from the Institute of Genetics, Zhejiang University published their collaborative research regarding the developmental delay and seizure manifested by aberrant mitochondrial tRNA metabolism in the journal Nucleic Acids Research on December 8.
In this study, the research team focused on FARS2 deficiency and illustrated the aberrant mitochondrial tRNA metabolism can lead to developmental delay and seizure, two major clinical phenotypes associated with FARS2 mutations.
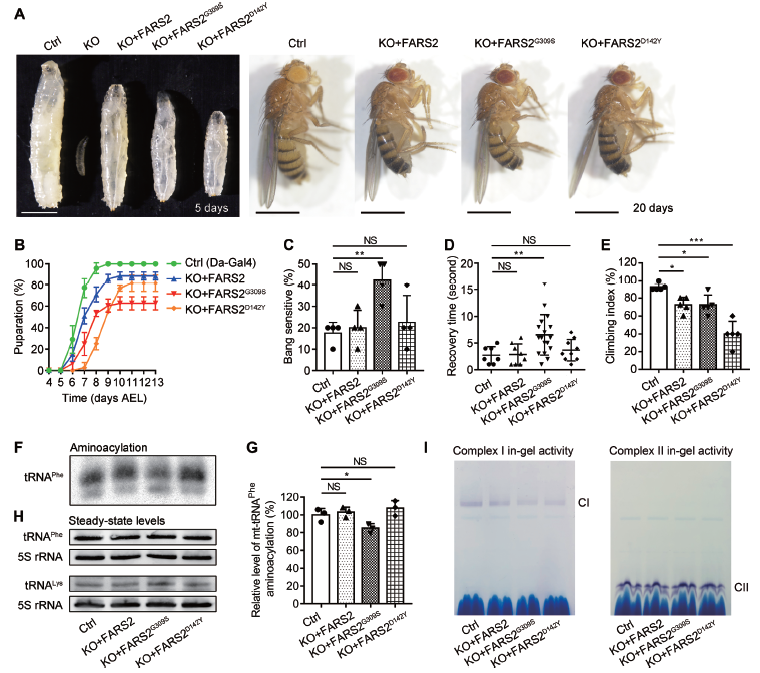
The researchers generated Drosophila FARS2 knockout and knockdown models and performed a series of experiments to confirm the validity of the model for studying mitochondrial tRNA synthetases related diseases. They found that FARS2 deficiency caused defects in mitochondrial tRNAPhe metabolism, translation, assembly and activity of oxidative phosphorylation system (OXPHOS) complexes. Moreover, the developmental delay and seizure observed in Drosophila FARS2 deficiency model accurately recapitulated many phenotypic features of human disease. The team also produced humanized fly models by introducing human wild type FARS2, p.G309S and p.D142Y pathogenic variants into Drosophila FARS2 mutants and analyzed the pathologic consequence of human disease-causing FARS2 p.G309S and p.D142Y mutations. These new findings suggest that proper control of mitochondrial tRNA metabolism is essential for mitochondrial function, revealing a novel genetic basis for mitochondrial disease.
"Our research discoveries will facilitate new understanding of pathogenesis of mitochondrial diseases and new therapies for treating seizures," said Ge.






