A group of scientists from our top-rated Chemistry Department and University of York have twisted molecules to their breaking point in order to challenge the understanding of chemical bonds.
Chemical bonding in molecules
Chemical bonding in aromatic molecules is key to the structure, stability and function of chemicals such as drugs and plastics.
For a molecule to be termed 'aromatic', some of its electrons must flow freely around a ring in its structure.
A classic example is benzene. Six of its electrons are shared around a ring of six carbon atoms.
Aromatic rings
Aromatic rings prefer to be flat. However, recent research has shown this isn't always the case. When aromatic rings are strained, they become twisted.
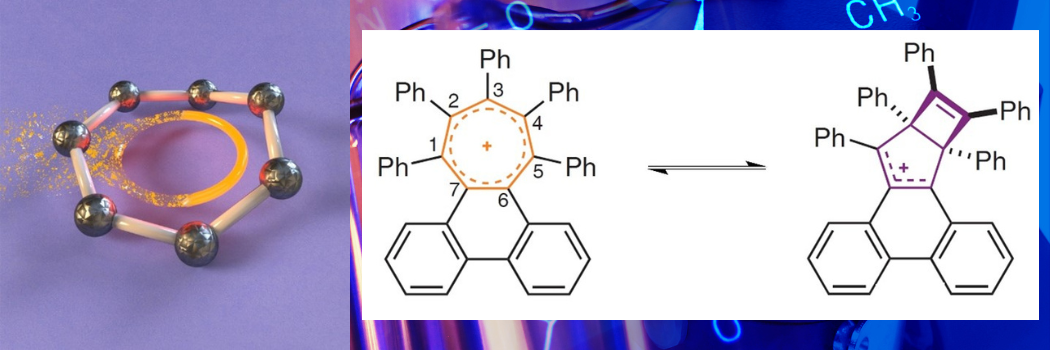
The researchers explored how far a ring can be twisted before its aromatic bonding breaks.
They achieved this by making overcrowded aromatic rings. Rather than benzene, they used tropylium, which shares electrons around a ring of seven carbon atoms.
Each of these carbon atoms can be functionalised and having seven attachment points in the ring rather than the six carbon atoms of benzene allowed the researchers to cram more groups around the edge of the aromatic ring, causing more strain.
The researchers found that low levels of overcrowding made the ring twist, but without breaking its aromatic bonding.
Remarkably, the molecule could be twisted by 45° from one end to the other.
By adding progressively larger groups around the edge of the ring, the team twisted the ring further, eventually causing the aromatic bonding to break.






