As an important carrier of material and energy metabolism, the ordinated modulation of organelle-associated molecules are vital for proper cellular and systemic functions. For example, sterol-regulatory element binding proteins (SREBPs), located on the surface of the endoplasmic reticulum (ER), can rapidly sense changes in intracellular lipid levels, modulate the rate of lipid synthesis to maintain cellular metabolic homeostasis. Ras-related GTP-binding proteins (RAGs) can recruit mTORC1 to the lysosomal surface for subsequent activation, thereby facilitating anabolism to meet the demand of proliferation. Delving into the dynamic regulatory mechanisms of bio-functional molecules at organelle contact sites could provide unique insights into diseases like cancers.
Cholesterol, an essential lipid for eukaryotic cells, contributes to the structural basis of biological membranes and acts as a signaling molecule to regulate cellular events. On one hand, excess intracellular cholesterol supplies membrane material for cell proliferation and metastasis. On the other hand, whose deregulation interferes cell signaling and drives oncogenes expression, which accelerates the progression of various malignant diseases such as hepatocellular carcinoma. In recent years, it has been reported that cholesterol is dynamically distributed in intracellular membrane structures to ensure functions for different organelles. However, the role of cholesterol-mediated organelle interactions in tumor-related signaling and metabolic dysregulation remains to be elucidated. Hence, further elucidating the regulation and function of cholesterol will shed light on the discovery of attractive targets for cancer treatment.
Over 90% of human nucleic acid transcripts are non-coding RNA, and the long non-coding RNA (IncRNA), which takes up the largest part, plays a vital role in modulating signaling, cellular metabolism and tumorigenesis. Recently, the team led by Prof. LIN Aifu from the Zhejiang University College of Life Sciences have comprehensively interrogated the global subcellular RNA landscape and identified the mitochondria-localized lncRNA growth-arrest-specific 5 (GAS5) as a tumor suppressor in maintaining cellular energy homeostasis (Nature Metabolism, 2021). However, the roles of RNAs as messengers in or regulators of cellular organelle communication and metabolic processes remains to be clarified, which would expand our current cognition of the roles of lncRNAs.
On August 22, the research team published an article entitled "Long non-coding RNA SNHG6 couples cholesterol sensing with mTORC1 activation in hepatocellular carcinoma" in the journal Nature Metabolism. This study reveals that lncRNA SNHG6 as an important player in the progression from non-alcoholic fatty liver disease (NAFLD) to hepatocellular carcinoma (HCC) by linking cholesterol sensing with mTORC1 signaling.
On the strength of previous studies, LIN Aifu et al. uncovered that lncRNA SNHG6 is distributed to ER-lysosome contact sites to accelerate NAFLD-HCC progression. SNHG6-pulldown MS analysis was employed to explore whose linkers in ER and lysosomes. The results showed that cholesterol promotes the interaction of SNHG6 with FAS-associated factor 2 (FAF2) and the mechanistic target of rapamycin kinase (mTOR). Subsequently, they discovered that cholesterol promotes SNHG6-FAF2-mTOR complex formation to regulate mTORC1 lysosomal recruitment and kinase activation at organelle contact sites. In addition, they clarified that cholesterol directly binds to FAF2 at residue 354, independent of the unsaturated-fatty-acid-binding region in FAF2, and cholesterol changes the conformation of FAF2 to assemble the SNHG6-FAF2-mTOR complex. These findings demonstrated that SNHG6 accelerates NAFLD-HCC progression via positive feedback with cholesterol.
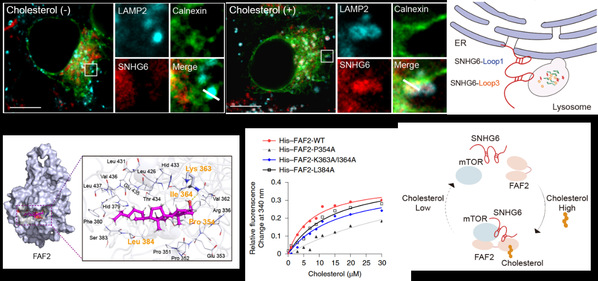
Cholesterol regulates SNHG6-FAF2-mTOR complex formation.
To further determine the regulatory role of SNHG6 in the NAFLD-HCC progression, LIN Aifu et al. constructed cholesterol-induced NAFLD-HCC mouse models. They found that SNHG6 overexpression in mice livers promotes cholesterol-induced mTORC1 activation and exacerbates NAFLD-HCC progression. Moreover, loss of SNHG6 inhibits mTORC1 activation in tumor tissues of the patient-derived xenograft (PDX) models, suggesting that SNHG6 can serve as a potential target for liver cancer treatment.
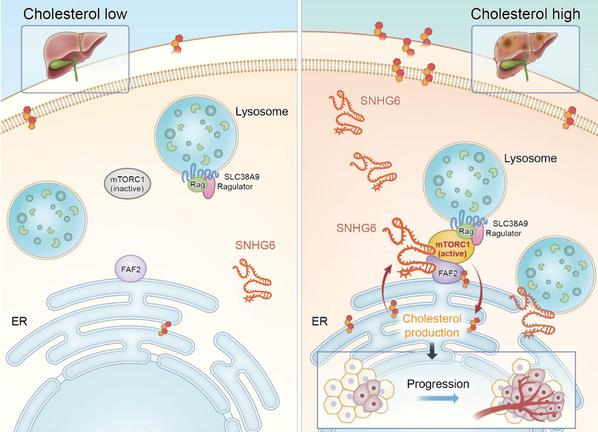
Illustration of SNHG6-modulated cholesterol-dependent mTORC1 signaling at ER-lysosome contact sites in hepatoma.
Together, these findings highlight the crucial role of organelle-associated lncRNA in organelle communication, nutrient sensing, and kinase cascades, thereby opening up new avenues for tumor treatment at the subcellular level.






