An international research collaboration, led by Prof. Dr. Robert Grosse (Centre for Integrative Biological Signalling Studies and Institute of Clinical and Experimental Pharmacology and Toxicology, University of Freiburg), Dr. Libor Macurek (Institute of Molecular Genetics, Czech Academy of Sciences, Prague) and Dr. Zdenek Lansky (Institute of Biotechnology, Czech Academy of Sciences, Prague) has uncovered a new mechanism of the crosstalk between microtubules and actin cytoskeleton during cell division and revealed unique characteristics of the previously unexplored protein FAM110A. These breakthrough findings significantly enhance the understanding of a critical process that is relevant in the occurrence of developmental disorders and cancer. The study has been published in the journal Proceedings of the National Academy of Sciences.
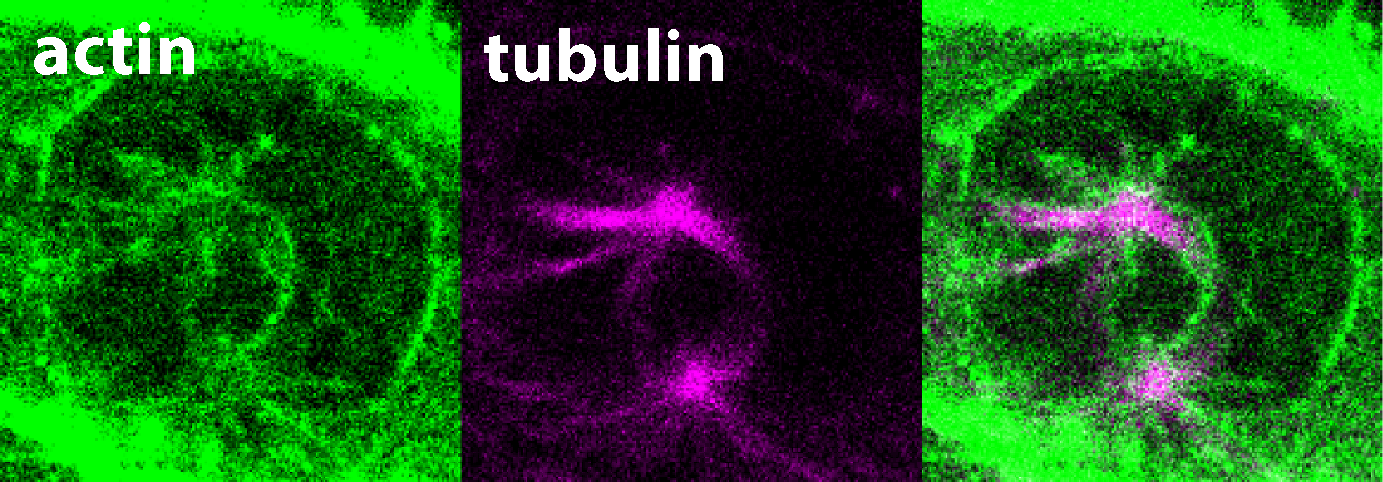
Excerpt from a film of living cells in the early stage of mitosis, the actin is colored green, the microtubules are colored purple. Photo: Robert Grosse / Universität Freiburg
The crucial role of FAM110A in the proper formation of spindle actin
Precise segregation of the genetic information into daughter cells is essential in all tissues of our bodies. This process needs to be tightly regulated in space and time to prevent developmental abnormalities. It has been known for decades that chromosomes attach to a bipolar structure called mitotic spindle that is composed of so-called microtubules. As mitosis proceeds - the process by which a cell divides its nucleus and genetic material to produce two identical daughter cells, ensuring equal distribution of chromosomes - the attached chromosomes are pulled along the microtubule railways to the daughter cells. Until recently, scientists believed that actin filaments are needed only for the final step of daughter cell separation and the role of actin cytoskeleton in mitosis has long been neglected. In their latest study, the research team now demonstrates that the previously unexplored protein FAM110A has unique properties that enable it to bind actin and microtubules at opposite ends, specifically at the poles of the mitotic spindles. Microscopic analysis revealed the formation of highly dynamic actin filaments around the spindle poles which precede and guide the growth of spindle microtubules. In the absence of FAM110A, proper formation of spindle actin was disrupted, leading to severe impairment in chromosomal segregation. Accordingly, the study discloses a crucial molecular link between the two primary cytoskeletal networks during mitosis. This breakthrough paves the way for future investigations into how FAM110A and related proteins found in human cells prevent genome instability and the development of cancer.
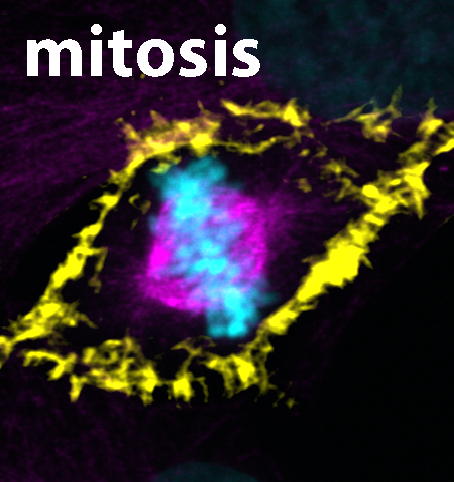
A fixed human cell during mitosis. The DNA is colored blue, the microtubules are purple and the actin is yellow. Photo: Libor Macurek
- Original Publication: Cecilia Aquino Perez, Mahira Safaralizade, Roman Podhajecky, Hong Wang, Zdenek Lansky, Robert Grosse and Libor Macurek (2024): FAM110A promotes mitotic spindle formation by linking microtubules with actin cytoskeleton. In: Proceedings of the National Academy of Sciences. DOI: https://doi.org/10.1073/pnas.2321647121
- Prof. Dr. Robert Grosse is member of the Cluster of Excellence CIBSS - Centre for Integrative Biological Signalling Studies and director of Division I at the Institute of Experimental and Clinical Pharmacology at the Medical Faculty of the University of Freiburg.
- This study was supported by the German Research Foundation (DFG).






