Max Planck researchers identify epithelial stem cells that control the growth of the thymus at different stages of life
Many immune cells crucial for our immune system develop in a small organ next to our heart: the thymus. With age, however, the thymus shrinks, and the number of effective immune cells declines. Max Planck Research groups from Freiburg and Würzburg have now identified the processes that control the development and composition of thymic tissue throughout life. Moreover, they discovered new potential therapeutic approaches for slowing down age-related thymus shrinkage and counteracting autoimmune diseases.

The fine tissue structure of the stimulated thymus organ does not differ from that of an unstimulated organ. As a typical sign of a well-functioning thymus, the marginal zones (red) and inner zones (green) are sharply separated from each other.
© MPI of Immunobiology & Epigenetics, Boehm
The thymus is a crucial organ of the immune system. In the thymus, the well-known T cells mature: As killer cells, they recognize and destroy virus-infected or malignant cells, and as so-called helper T cells they assist the body in antibody formation. In the last decades, Thomas Boehm's research group at the Max Planck Institute of Immunobiology and Epigenetics in Freiburg has identified the genetic switches required for T cell maturation in the thymus. An essential component for this process are so-called thymic epithelial cells, which attract T cell precursors and induce them to mature into fully functional T cells. During this development, T cells are instructed to distinguish diseased from healthy cells and foreign material from the body's own cells, thus enabling them to detect and eliminate unwanted structures and prevent autoimmune diseases. Earlier work in the Boehm laboratory had shown that the two main types of thymic epithelium arise from bipotent progenitor cells. However, it was unclear whether there is more than one type of progenitor, and it was unknown into how many subforms the progenitors differentiate.
Molecular family tree analysis identifies progenitor cells
In collaboration with the laboratory of Dominic Grün (previously at the MPI of Immunobiology and Epigenetics in Freiburg, now Max Planck Research Group at the University of Würzburg), a specialist in single-cell RNA analysis, the researchers have now succeeded in describing the unexpected diversity of thymic epithelial cells at the transcriptional level. Algorithms developed in Grün's laboratory for the precise description of differences in the gene activity of individual cells made it possible to identify potential precursor cells. In a second step, the researchers experimentally verified the predictions using a "barcoding" system developed in the laboratory of Thomas Boehm using CRISPR gene editing. The barcoding method allows to assign a molecular signature to precursor cells, which is then carried along by all cells that emerge from the precursors. In this way, the researchers derived a family tree of epithelial cells.
After a long period of method development that was marked by many setbacks, Anja Nusser from the Boehm lab and Sagar from the Grün lab jointly succeeded in developing a method that connects information from the phylogenetic tree with the molecular characteristics of individual cells. As a result, for the first time it became possible to study the development of thymic epithelium at different ages in equisite molecular detail. This kind of analysis is of particular interest to immunologists because the thymus is subject to significant changes during life. Rapid organ growth and massive T-cell production are characteristic of the early developmental stages. In contrast, there is a gradual loss of functional thymic epithelial cells in old age and, therefore, decreased T-cell production. These age-related changes are associated with a reduced immune function.
Successive activities of the progenitor cells determine the composition of the thymus
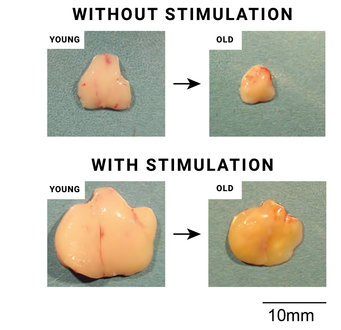
Massive expansion of the stimulated thymic organ even in young mice, which is mainly preserved in old age.
© MPI of Immunobiology & Epigenetics, Boehm
The researchers identified two bipotent progenitor populations of the thymic epithelium in their analysis. An "early" progenitor population takes over the primary role in the thymus formation during embryonic development. While in the juvenile organism, a subsequent "postnatal" progenitor population significantly determines the continued thymus formation in adulthood. Interestingly, the progenitor populations' temporal order modulates the thymic epithelium's composition.
At early time points, mainly cortical thymic epithelial cells are formed, which primarily contribute to the production of T cells. At later time points, the primary output is on medullary thymic epithelial cells. They ensure that no self-reactive T cells are released from the thymus into the body and thus, contribute significantly to protection against autoimmunity.
New therapeutic approaches to increase thymic function
The sophisticated combination of transgenic animal models from the Boehm laboratory with state-of-the-art methods of single-cell profiling of the Grün group allowed the researchers to examine the effect of increasing the proliferation of thymic epithelial cells. It was of particular importance to determine whether early stimulation of the thymus with a dedicated growth factor leads to undesirably faster consumption of stem cells and thus to premature shrinkage of the thymus. However, the data obtained by the researchers suggest that this is not the case. "The stimulated thymus of an old mouse is still larger than that of an unstimulated young mouse. Moreover, the fine tissue structure of the stimulated thymus shows the typical structure of cortical zones and medullary areas inside the organ," says Max Planck director Thomas Boehm. These results lay the foundation for developing new therapeutic approaches to correct age-related thymic shrinkage and treat T-cell-dependent autoimmune diseases.
TB/MR






