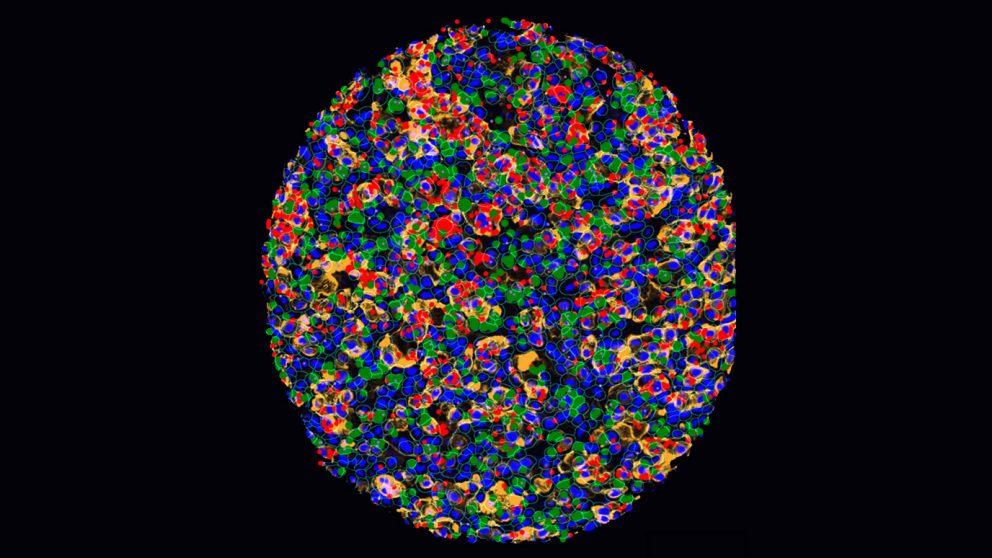
In a new study, researchers from The University of Texas MD Anderson Cancer Center created the largest single-cell atlas of brain metastases from renal cell carcinoma (RCC) with matched primary and extracranial metastases, enabling the discovery of key biological mechanisms driving an immunosuppressive tumor microenvironment in the brain distinct from that of the kidney or other metastatic sites. Findings were presented today at the American Association for Cancer Research (AACR) Annual Meeting 2023.
The study, led by Elshad Hasanov, M.D., Ph.D., medical oncology fellow at MD Anderson, provides further insights into how and why RCC, the most common type of kidney cancer, is more difficult to treat when it metastasizes to the brain compared to other sites. The data also point to several unique therapeutic targets that could be explored to improve the anti-tumor immune response.
"Brain metastases are, by far, the most challenging complication of kidney cancer. We've seen systemic therapies work in treating primary tumors and other metastatic sites, but they are not as effective for brain metastases. The current standard of care, radiation treatment and surgery, helps to treat the brain metastases but does not prevent new metastases," Hasanov said. "We now see it's not just because of the blood-brain barrier, but it also is due to interactions between the tumor and other immune and stromal cell populations, creating an immunosuppressive microenvironment in the brain that allows tumors to somehow escape immunotherapy."
Many patients with RCC are treated with immune checkpoint inhibitors, but nearly 30% will eventually develop brain metastases as the progressing disease site. Due to poor treatment responses, patients with RCC brain metastases have a poor prognosis, underscoring a need to understand the mechanisms driving the brain as an immune-privileged organ that allows for tumor growth and spread even after immunotherapy.
Researchers at Emory University, Hacettepe University and MD Anderson collaborated to collect matched samples from primary kidney tumors as well as extracranial and brain metastases from patients who also underwent surgery. These consisted of frozen tissue samples from RCC patients, including 14 brain metastases, eight matched primary kidney tumors and five matched extracranial metastases. An additional 57 formalin-fixed paraffin-embedded (FFPE) brain metastases samples were included in the analysis.
The researchers performed single-nucleus RNA sequencing on nearly 200,000 cells to characterize gene expression within the samples. Using RNA spatial molecular imaging techniques, they mapped interactions between major cell types found in the tissues.
Tumor microenvironment of brain metastases has unique immunosuppressive features
Through their analysis, the researchers discovered that brain metastases had a strikingly immunosuppressive tumor microenvironment compared with primary tumors or extracranial metastases.
Brain metastases had greater infiltration of neuronal and glial cells, driving inflammatory responses that appear to suppress anti-tumor activity by binding with immune cells via known immunosuppressive ligand-receptor interactions. Brain metastases also had fewer proliferating T cells, memory B cells, dendritic cells and monocytes. Macrophages in the brain had more highly expressed immune-suppressing M2 gene signatures, which promote cell proliferation and repair.
Additionally, the brain metastases had higher activity of the VEGFR and FGFR4 growth-promoting proteins, higher levels of immune checkpoint proteins and an enrichment of various targets and pathways - including MYC genes - all of which allow the cancer cells to thrive in that microenvironment. Interestingly, the presence of naïve/memory T cells in the brain was associated with favorable overall survival after surgery, making this a potential prognostic marker.
Based on these results, Hasanov and colleagues plan to pursue further preclinical and clinical trials to test various combination therapies against VEGFR and FGFR4 (lenvatinib) and other identified targets in combination with immune checkpoint inhibitors (pembrolizumab) for patients with RCC brain metastases.
"There are communication highways between neuronal cells and other cell populations that do not exist in the kidney and other metastatic sites, but they are very dominant in the brain. It's a whole other world with different tumor- and host organ-driven players and communications," Hasanov said. "The beauty of single-nucleus sequencing and spatial transcriptomics is that it allows us to look deeper and create snapshots of these cells and their location so we can better understand their biological value at the protein and RNA level. This helps us to identify potential therapeutic targets and to work toward designing therapies that can improve patient outcomes."
The study was supported by the Kidney Cancer Association Young Investigator Award 2021, the International Kidney Cancer Coalition Cecile and Ken Youner Scholarship Award 2021, the Society for Immunotherapy of Cancer - NanoString Technologies Single Cell Biology Award 2022 and Cancer Prevention and Research Institute of Texas (CPRIT) (RP180684). Hasanov has received research funding to his institution from the Conquer Cancer Foundation, the Kidney Cancer Association, the International Kidney Cancer Coalition, and the Society for Immunotherapy of Cancer; honoraria from Targeted Oncology; and has served an advisory role for Telix Pharmaceuticals. A complete list of collaborating authors and their disclosures can be found here.