Leiden chemists, together with colleagues at the University of York (UK) and Technion (Israel) have discovered a small, sugar-like molecule that maintains the integrity of tissue around a tumour during cancer. This molecule prevents tumour cells from spreading from the primary cancer site to colonise other sites in the body. The multidisciplinary international team published their research on July 26 in the renowned scientific journal Proceedings of the National Academy of Sciences (PNAS).
Metastasis, the spread of cancer cells to distant sites in the body, is what makes cancer so lethal. Metastasis formation depends on the ability of cancer cells to detach from the primary tumour site, and invade through blood vessel walls and tissue barriers to reach secondary sites of growth. This metastatic invasion process requires biological molecules called enzymes, which digest proteins and sugars in the space around cells, enabling cancer cells to pass through the subsequent gaps.
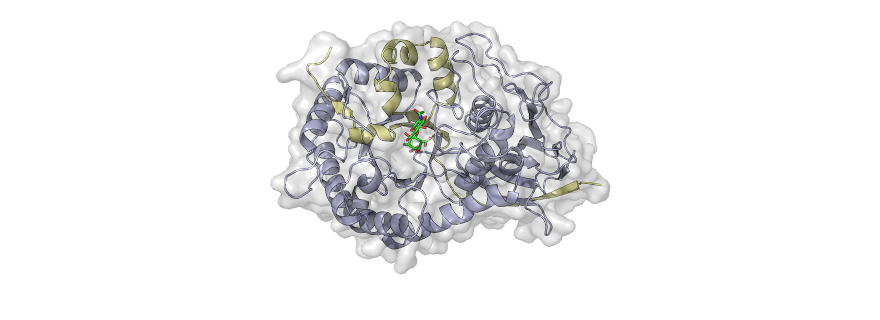
One abundant class of sugar that surrounds cells are the heparan sulfates, which are long chain-like molecules that help stabilise the integrity of the extracellular space. Heparan sulfate sugars are digested by an enzyme called heparanase, which acts to chop up the 'chains' and thereby weaken the space around cells. Metastatic cancer cells produce large amounts of heparanase enzyme, which helps them to spread around the body. Inhibiting heparanase is therefore a major target for anti-cancer therapy.
Contact with colleagues in Israel
Leiden chemist Hermen Overkleeft designs and makes molecules, and he has been working on enzyme inhibitors for years, together with Gideon Davies and Liang Wu from the University of York. 'When we came across something that could be relevant to cancer therapy, we contacted Israel Vlodavsky and his colleagues in Haifa, who specialise in the role of heparanase in tumour growth and metastasis.'
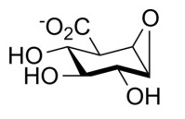
Together, they developed and tested a new sugar-like molecule that reacts with the enzyme heparanase. Once attached, the heparanase enzyme is unable to bind or cut heparin sulfate sugar chains around cells. In this way, the tissue around cells remains firm and inaccessible to dislodged cells.
Tested in three types of cancer
The colleagues from York showed how the enzyme inhibitor inhibits heparanase, and together they are now looking at how the molecule can be further improved. Overkleeft: 'Can we make it even more powerful, and even more selective? But also: can the molecule be fine-tuned in such a way that it actually starts behaving like a medicine?' The colleagues in Haifa (Technion) already studied the new molecule in mouse models of lung cancer, breast cancer and blood cancer. The results are promising and the institutes involved have already applied for a patent on the molecule.
Clinical application is still uncertain
It is still too early to determine whether the new molecule will reach clinical application. 'Now we have to find out whether the compound is stable, safe for the human body, ends up in the right place in sufficient quantities, and so on. That takes a couple of years, it may come to nothing and someone has to be willing to take that financial risk.'
Overkleeft believes that this molecule certainly deserves a chance. 'Our molecule is one of the few agents that can inhibit heparanase tightly and specifically. Small, well-defined molecules like this one may be easier to develop into a clinical drug than the large, heterogeneous polysaccharides that have been tried up to now.'
Text: Rianne van Lindhout
Article: Website PNAS






