RICHLAND, Wash.-Scientists have developed a way to detect tiny amounts of hard-to-detect explosives more than eight feet away, reducing the need to swipe clothing, luggage or other materials.
The method, published in the chemistry journal Talanta, detects trace amounts of explosives like nitroglycerin and RDX-the explosive in C-4-through the air at extremely low levels within seconds. Such substances give off just a few precious molecules-but scientists have nonetheless created ways to detect them. Their explosives detection equipment is so sensitive, it recognizes the smallest wisp of an explosive in an area full of common air molecules like nitrogen and oxygen.
The team can identify explosives at a level of less than 10 parts per quadrillion-like identifying a single pine needle from all the pine trees in the state of Washington. Put another way, it's the equivalent of being able to pluck out a single coin from a stack of pennies more than 17 million times higher than the height of Mount Everest.
The recent research by scientists at the Department of Energy's Pacific Northwest National Laboratory builds on more than a decade of work detecting vapors from explosives and other materials. In the past, the team developed methods to detect explosives from about half an inch away. The latest explosive detection technology recognizes the same substances from two to eight feet away, depending on the material.
"Previously, we could detect these explosive materials at a distance of the width of your finger. Now, our technology reaches the length of your arm and beyond," said Robert Ewing, the PNNL chemist who leads the team.
The technology has been licensed to BaySpec Inc., a Silicon Valley instruments manufacturing company that specializes in spectral sensing for applications ranging from semiconductors and pharmaceuticals to defense and security. The company plans to have a commercial product based on the PNNL technology for the detection of explosives and narcotics available in 2025.
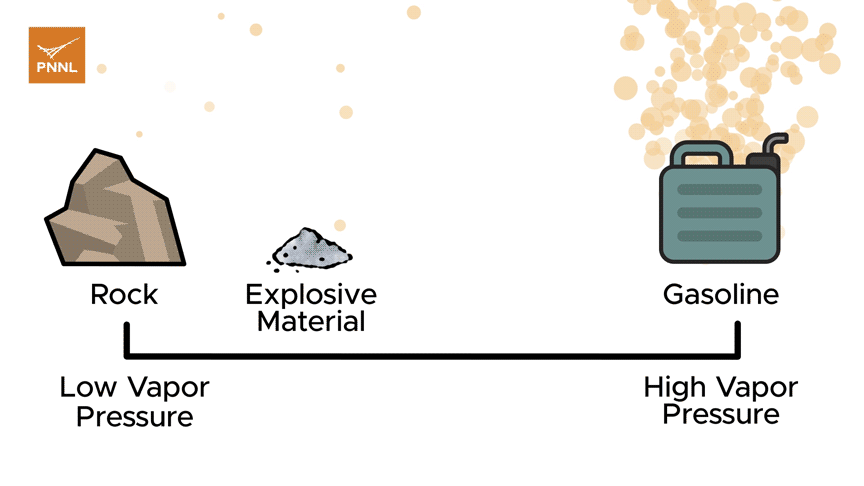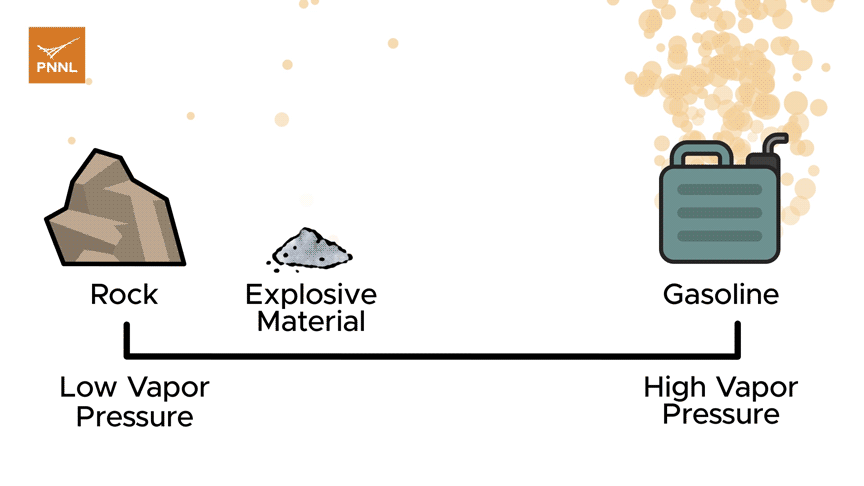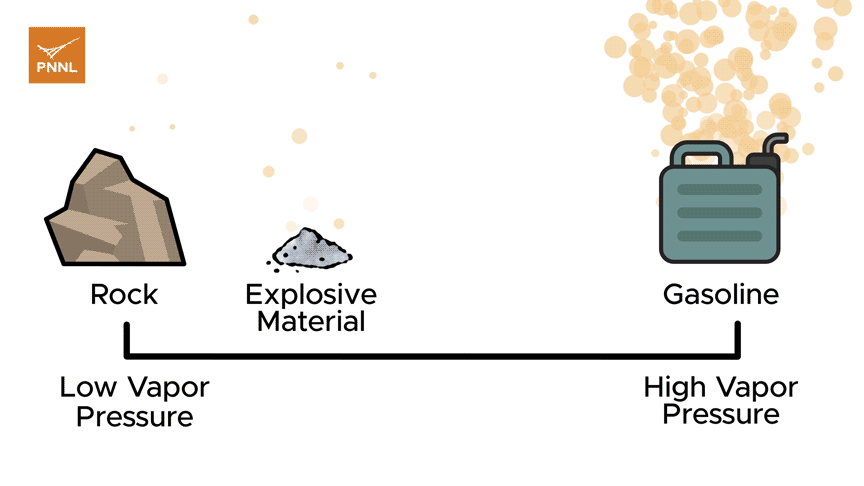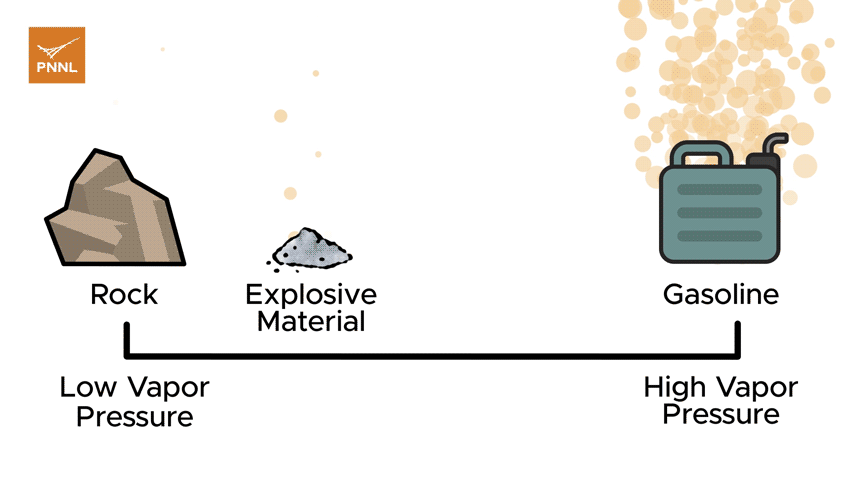
Lower vapor pressure: no barrier to explosives detection
Ewing's team is tackling materials that have low vapor pressure-they don't evaporate quickly. They release so few molecules into the air that it's hard to capture and analyze them. That's different from materials with higher vapor pressure, like gasoline-gasoline is volatile and its molecules become airborne quickly, making it easy to detect.
In the latest paper, the team detected low-vapor explosives like nitroglycerin and RDX-another common explosive-from several feet away. In previous work, the team detected explosives like tetryl, PETN and TNT through the air, as well as drugs like fentanyl, cocaine and methamphetamine. The team believes the new approach, known as standoff detection, can also be used for additional explosives, drugs and other chemical threats.
"We hope this technology can be deployed to keep people safe from explosive threats," said Ewing.
Part of the improvement in standoff detection distance is due to a powerful, handheld air sampler developed at the University of Washington that draws in approximately 300 liters of air per minute. That allows the PNNL scientists to collect all the air they need in just 5 to 10 seconds to detect materials with lower vapor pressure.
The air is drawn through a filter that collects the vapors, which are then delivered to an atmospheric flow tube and detected by a mass spectrometer.
A key to the sensitive detection is the approximately two-foot-long device called an atmospheric flow tube where molecules are ionized before they're sent to the mass spectrometer. The distance of this tube allows for more time (seconds vs. milliseconds) for the target molecules to be ionized, which increases the sensitivity of detection.
With the atmospheric flow tube, the team can identify explosives at a level of less than 10 parts per quadrillion.

The work builds on the team's previous success, which included an R&D 100 Market Disruptor Award. The team participated in the Trace Explosives & Drug Detection Workshop in Ireland June 24-28 to discuss related work.
The work is part of a broad PNNL program in explosives detection. This research was funded by the Department of Homeland Security Science and Technology Directorate. Corresponding authors of the paper are Megan Nims, Elizabeth Denis, and Ewing. Additional authors are PNNL scientists Garret Hart, Nancy Escobedo, Shannon Schrader and R. Shane Addleman, and Igor Novosselov of the University of Washington.






