A new study led by the Qingdao Institute of Bioenergy and Bioprocess Technology of the Chinese Academy of Sciences has identified significant safety risks in sodium-ion batteries (SIBs), especially compared to lithium-ion batteries (LIBs).
Published in Energy & Environmental Science, the study reveals that SIBs are more prone to thermal runaway than previously assumed, challenging their perceived safety despite cost and resource advantages.
Over the past decade, SIBs have achieved energy densities of up to 160 Wh/kg and cycle lives exceeding 4,000 charge-discharge cycles. Despite these advancements, safety remains a critical concern. This study highlights the role of sodium clusters in hard carbon (HC) anodes, which can trigger early thermal runaway. These clusters exhibit electronic activity surpassing even metallic sodium, creating localized "electronic storms" within the battery.
Prof. CUI Guanglei, a co-corresponding author of the study, explained, "When the battery reaches critical charge levels, sodium clusters lower the self-heating onset temperature to as low as 92°C, triggering thermal runaway much earlier than in LIBs." These clusters act as catalysts, accelerating electrolyte decomposition and intensifying thermal runaway.
Using solid-state nuclear magnetic resonance (ssNMR) spectroscopy, the researchers investigated sodium clusters at the quantum scale. They found that these clusters exhibit significant metallicity, with more conduction electrons at the Fermi energy level than bulk metallic sodium, making them highly reactive and accelerating thermal runaway.
Unlike LIBs, where exothermic reactions are independent of the state of charge (SOC), SIBs exhibit a strong dependence between reaction onset and SOC. Sodium clusters form at elevated SOCs, lowering the onset temperature for self-heating well before the SEI decomposition typically seen in LIBs. The study suggests that even during normal operation, SIBs may face safety risks comparable to those associated with sodium plating in overcharged batteries.
These findings bridge a critical knowledge gap in understanding the connection between cell safety and sodium storage microenvironments. The research team suggests that replacing liquid electrolytes with solid-state materials could significantly reduce thermal runaway risks, offering a safer alternative for future energy storage.
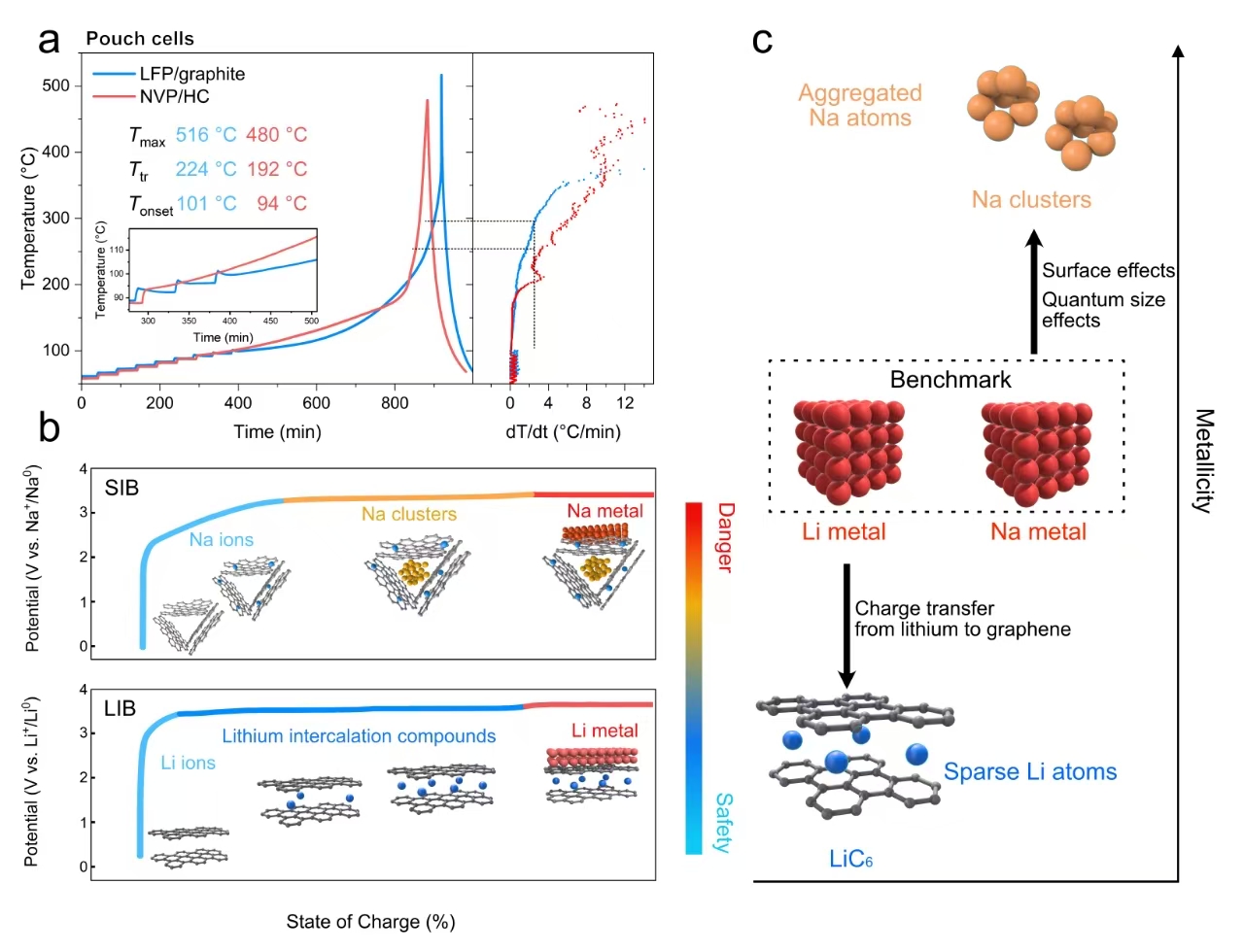
(a) Comparison of thermal runaway modes in sodium-ion and lithium-ion pouch cells. (b) Evolution of alkali metal ions in hard carbon and graphite anodes. (c) Comparison of sodium clusters with LiC6 and their corresponding metallic forms. (Image by the research team)






