EMBL researchers have created an engineered uterus that allows a closer look at a mouse embryo's development and its interactions with the uterine environment
The development of a complex organism from a single cell demands the precision and coordination of an orchestra performing a symphony.
We have come a long way in understanding embryonic development, but there are still many questions surrounding the critical early stages of this intricate process, particularly the interaction between the embryo and the mother's uterus during the implantation phase.
Understanding this process more deeply can provide fundamental insights into developmental biology, reproductive health, evolution, and much more. However, studying it is challenging, particularly due to the inaccessibility of the uterus for detailed investigations.
To tackle this obstacle, Vladyslav Bondarenko, who ran the project when he was a PhD student in the research group of Takashi Hiiragi, former Group Leader at EMBL and currently a group leader at Hubrecht Institute, the Netherlands, collaborated with the Lutolf Group at the Swiss Federal Institute of Technology, Lausanne (EPFL), along with the Ellenberg, Erzberger, and Kreshuk groups at EMBL Heidelberg, and the Uhlmann group at EMBL-EBI. Taking an innovative approach, the teams utilised engineered systems to closely mimic natural conditions during development.
By carefully controlling the shape, stiffness, and adhesion properties of this synthetic environment, they built the '3E-uterus' - an ex vivo engineered uterine environment made of a jelly-like and transparent material, with a cylindrical 3D structure.
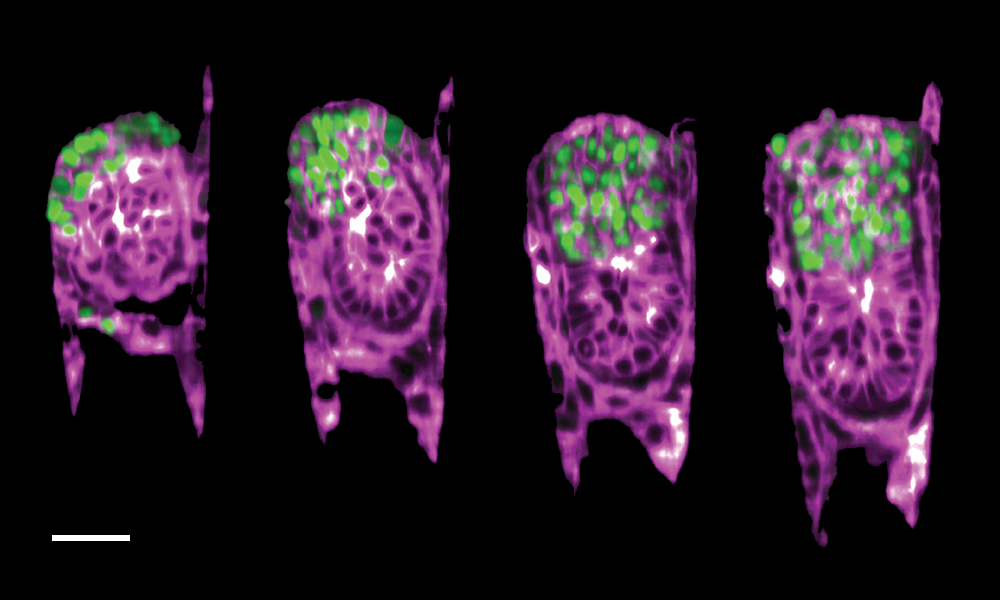
This image taken by Bondarenko and Dimitri Kromm, former PhD student in the Ellenberg group, shows the growth of a mouse embryo inside the 3E-uterus. This 12-hour time-lapse imaging of a two-day-old embryo captures the very first stages of its development. To obtain it, the researchers combined their artificial uterus with light-sheet microscopy - a cutting-edge technology for imaging which allowed them to have a close look at the dynamic behaviour of a developing mouse embryo.
As seen in the image, the extraembryonic ectoderm cells (marked in green) - specialised cells that provide structural support to the embryo and later contribute to the formation of the placenta - slowly fold inwards into the growing embryo (purple). This process, called invagination, is a crucial part of this very early stage of development and helps the embryo reach its proper shape and internal structure.
"We were able to observe the mouse embryo's implantation process, which resembles the physics of capillarity - the phenomenon is akin to how water droplets adhere to surfaces due to surface tension forces," said Anna Erzberger, leader of the EMBL Heidelberg group responsible for theoretically describing the embryo's behaviour in this controlled setting. She noted the similarity of this system to wetting-type problems in physics. "By merging the realms of biology and physics, we gain valuable insights into how the embryo changes during this process. This engineered environment simplifies the physics involved, making it mathematically tractable and enabling a deeper understanding."
With the 3E-uterus system, researchers can not only observe the otherwise inaccessible complex dance between the mouse embryo and the mother's uterus, but also open up new possibilities for studying such interactions in the field of reproductive medicine.
"With this, we only begin understanding the complex embryo-uterine communication," said Bondarenko. "The mammalian uterus can be engineered further to incorporate uterine tissue structure and dynamics across the scales, with many exciting challenges and findings ahead."






