Liposuction and plastic surgery aren't often mentioned in the same breath as cancer.
But they are the inspiration for a new approach to treating cancer that uses engineered fat cells to deprive tumors of nutrition.
Researchers at UC San Francisco used the gene editing technology CRISPR to turn ordinary white fat cells into "beige" fat cells, which voraciously consume calories to make heat.
Then, they implanted them near tumors the way plastic surgeons inject fat from one part of the body to plump up another. The fat cells scarfed up all the nutrients, starving most of the tumor cells to death. The approach even worked when the fat cells were implanted in mice far from the sites of their tumors.
Relying on common procedures like this could speed its use as a new form of cellular therapy.
"We already routinely remove fat cells with liposuction and put them back via plastic surgery," said Nadav Ahituv , PhD, director of the UCSF Institute for Human Genetics and professor in the Department of Bioengineering and Therapeutic Sciences. He is the senior author of the paper, which appears Feb. 4 in Nature Biotechnology . "These fat cells can be easily manipulated in the lab and safely placed back into the body, making them an attractive platform for cellular therapy, including for cancer."

Cold therapy sparks a new idea
Ahituv and his post-doc at the time, Hai Nguyen, PhD, were aware of studies that showed exposure to cold could suppress cancer in mice. One experiment even showed it could help a patient with non-Hodgkin lymphoma. Scientists concluded that the cancer cells were starving because the cold was activating brown fat cells, which use nutrients to produce heat.
But cold therapy isn't a viable option for cancer patients with fragile health. So, Ahituv and Nguyen turned to the idea of using beige fat, wagering that they could engineer it to burn enough calories, even in the absence of cold, to deprive tumors of the fuel they needed to grow.
Nguyen, who is the first author of the paper, used CRISPR to activate genes that are dormant in white fat cells but are active in brown fat cells, in the hopes of finding the ones that would transform the white fat cells into the hungriest of beige fat cells.
A gene called UCP1 rose to the top. Nguyen grew UCP1 beige fat cells and cancer cells in a "trans-well" petri dish. The cancer cells were on the bottom and the fat cells were above them in separate compartments that kept the cells apart but forced them to share nutrients. The results were shocking.
"In our very first trans-well experiment, very few cancer cells survived. We thought we had messed something up - we were sure it was a mistake," Ahituv recalled. "So, we repeated it multiple times, and we kept seeing the same effect."
The beige fat cells held sway over two different types of breast cancer cells, as well as colon, pancreatic and prostate cancer cells.
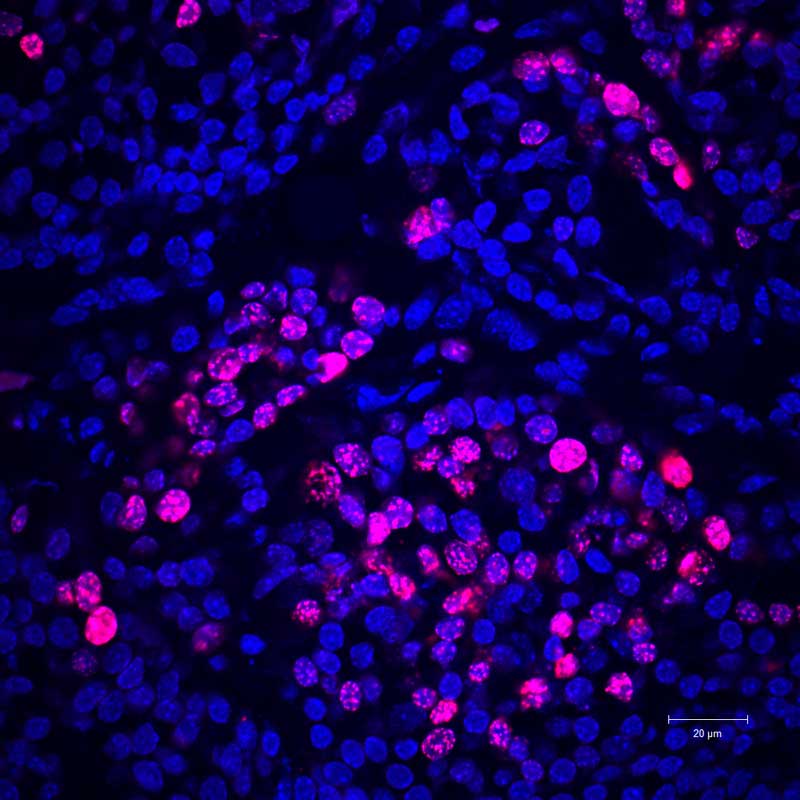
No fat cells: Actively-multiplying cancer cells (pink) are seen in a mouse predisposed to develop breast cancer.
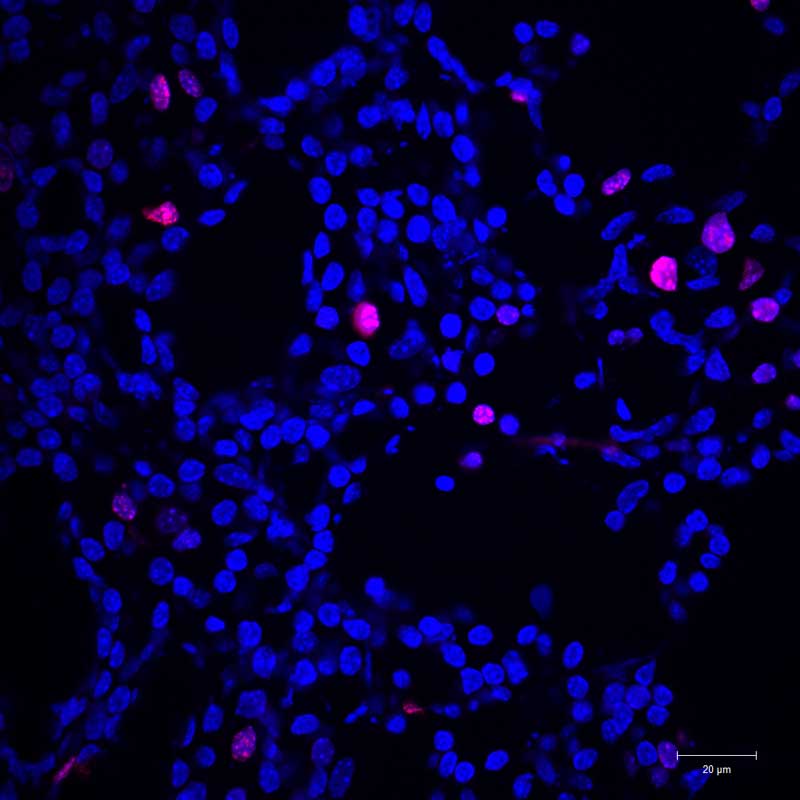
Fat cells are added: When fat cells were implanted in the breast, far fewer cancer cells were able to multiply.
Cancer is no match for hungry fat
But the researchers still didn't know if the beige fat cells would work in a more realistic context. So, they turned to fat organoids, which are coherent clumps of cells grown in a dish, to see if they could beat tumor cells when they were implanted next to tumors in mice. The approach worked against breast cancer, as well as pancreatic and prostate cancer cells. The cancer cells starved as the fat cells gobbled up all the available nutrients.
The implanted beige fat cells were so powerful that they suppressed pancreatic and breast tumors in mice that were genetically predisposed to develop cancer. It even worked when the beige fat cells were implanted far away from the breast cancer cells.

To see how they would work in human tissue, Ahituv and Nguyen teamed up with Jennifer Rosenbluth , MD, PhD, a breast cancer specialist at UCSF. Rosenbluth had amassed a library of breast cancer mastectomies containing both fat cells and cancer cells.
"Because the breast has a lot of fat, we could get fat from the same patient, modify the fat and grow it in a single trans-well experiment with that patient's own breast cancer cells," Ahituv said.
These same-patient beige fat cells outcompeted breast cancer cells in petri dishes - and when they were implanted together in mouse models.
Knowing that cancers have preferred diets, the researchers engineered fat just to eat certain nutrients. Certain forms of pancreatic cancer, for example, rely on uridine when glucose is scarce. So, they programmed the fat cells to eat just uridine, and they easily outcompeted the pancreatic cancer cells. This suggested that fat could be adapted to any cancer's dietary preferences.
A new approach to living cell therapy
Fat cells have many advantages when it comes to living cell therapies, according to Ahituv. They are easy to obtain from patients. They grow well in the laboratory and can be engineered to express different genes and take on different biological roles. And they behave well once they are put back into the body, not straying from the location where they're implanted and playing nice with the immune system. It's a conclusion supported by decades of plastic surgery.
"With fat cells, there's less interaction with the environment, so there's very little worry of the cells leaking out into the body, where they might cause problems," Ahituv said.
Fat cells can also be programmed to emit signals or carry out more complicated tasks.
And their ability to defeat cancer even when they are not right next to tumors could prove invaluable for treating hard-to-reach cancers like glioblastoma, which affects the brain, as well as many other diseases.
"We think these cells could also be designed to sense glucose in the bloodstream and release insulin, for diabetes, or suck up iron in diseases where there's excessive iron like hemochromatosis," Ahituv said. "The sky's the limit for these fat cells."






