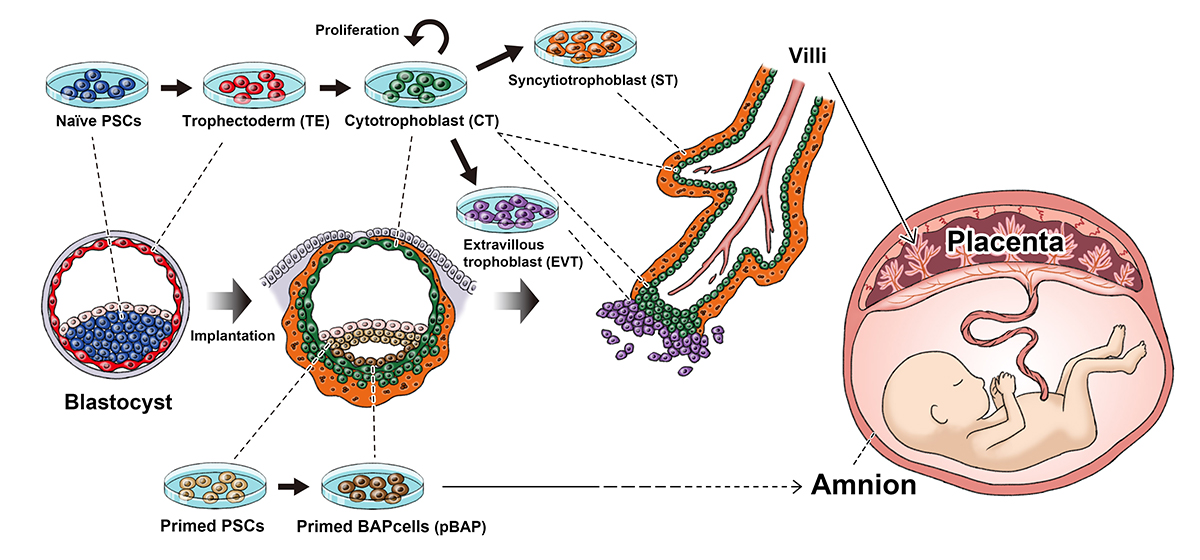
Naïve and primed PSCs (iPS cells and embryonic stem cells) represent different periods in development (before and after the embryo implants itself into the uterus). Naïve PSCs can become all the major cell components that make up the villi, which goes on to become the placenta. Primed PSCs cannot and instead form cells that lead to the amnion.
The Yasuhiro Takashima lab shows that naïve iPS cells can be induced to form all the stages that mimic early placenta development in humans.
Gynecologist Shingo Io knows that during birth there will not only be a baby leaving the mother's body. Joining the cries that bring joy to the room will be a silent entity, the placenta. Like the baby, this tissue only began to grow upon conception, but little is known about how the placenta develops inside the mother. A new study by Io, CiRA Junior Associate Professor Yasuhiro Takashima and colleagues reports how iPS cells can be used to study this development. The study is the first to show that iPS cells have this potential and will be invaluable for understanding the many factors that lead to miscarriage.
No matter how old or how big, all of us began as a fertilized egg, which is nothing more than a single cell. This one cell will grow into an embryo, which eventually leads to a fully formed human body. That one cell also grows into extra-embryonic tissue that becomes the placenta. While not part of the baby's body, the placenta is essential for healthy growth. Historically tossed aside like a bloody towel, it is now studied intensively.
"Abnormalities to the placenta can cause serious problems to the baby and mother like miscarriages, stillbirths and pre-eclampsia. We know much less about placenta development than embryo development," said Io, who is also a Ph.D. student and will graduate with publication of the paper.
The origins of the placenta are attributed to the region known as the trophoblast.
"Much of what we know about the trophoblast comes from mouse, but there are obvious species differences. We are trying to generate trophoblast cells from iPS cells," said Io.
Both human embryonic stem cells and iPS cells are thought to represent the state of the embryo just after pregnancy. Pregnancy begins approximately when the embryo attaches to the mother's uterine wall. Although the time between conception and pregnancy only amounts to several days, the embryo has already gone through significant changes during this time, meaning that iPS cells represent a stage in development too late to accurately model the trophoblast.
In 2014, Takashima found a way to modulate iPS cells so that they represent a stage prior to implantation.
"iPS cells can be grouped into naïve and primed states. Mouse iPS cells are naturally naïve, but human are primed. We found a way to reset the primed state to naïve state," he said.
This reset allows iPS cells to be used to study earlier stages of human development, because they can differentiate into more cell types.
Both naïve and primed human iPS cells can produce cells that resemble the trophoblast, but by comparing gene expressions of human embryos collected from other groups, the study found that only trophoblast cells made from naïve iPS cells had the potential to become placental tissue.
"The trophoblast cells made from naïve human iPS cells went on to produce cells for all stages of placenta development like the trophectoderm, cytotrophoblast, syncytiotrophoblast and extravillous trophoblast. Although we cannot make full placenta yet, this shows that they can be used to study the early stages of placenta development. Primed iPS cells showed characters of the amnion, not the placenta," said Io.
The transition from the trophectoderm to cytotrophoblast is thought to represent implantation into the uterine wall, which Io explains has important implications on studying healthy baby development.
"The pre-implantation to post-implantation stage is thought to be a major step in development," he said. "Most of our attention has been on understanding how the embryo grows into a body. But without healthy growth of the placenta, the baby will never go to term. Our study shows that iPS cells can be used to study this important step, avoiding the need to use human embryos."
Paper Details
- Journal: Cell Stem Cell
- Title: Capturing human trophoblast development with naïve pluripotent stem cells in vitro
- Authors: Shingo Io1,2, Mio Kabata1, Yoshiki Iemura1, Katsunori Semi1, Nobuhiro Morone3,
Atsutaka Minagawa4, Bo Wang4, Ikuhiro Okamoto5,6, Tomonori Nakamura5,6,7, Yoji Kojima1,5,6,
Chizuru Iwatani8, Hideaki Tsuchiya8, Belinda Kaswandy1, Eiji Kondoh2, Shin Kaneko4, Knut Woltjen1, Mitinori Saitou1,5,6, Takuya Yamamoto1,6,9,10, Masaki Mandai2, and Yasuhiro Takashima1 - Author Affiliations:
- Department of Life Science Frontiers, Center for iPS Cell and Application (CiRA), Kyoto University, Kyoto, Japan
- Department of Gynecology and Obstetrics, Kyoto University School of Medicine, Kyoto, Japan
- MRC Toxicology Unit, University of Cambridge, Cambridge, United Kingdom
- Department of Cell Growth and Differentiation, Center for iPS Cell and Application (CiRA),
Kyoto University, Kyoto, Japan - Department of Anatomy and Cell Biology, Kyoto University School of Medicine, Kyoto, Japan
- Institute for Advanced Study of Human Biology (WPI-ASHBi), Kyoto University, Kyoto, Japan
- The HAKUBI Center for Advanced Research, Kyoto University, Kyoto, Japan
- Research Center for Animal Life Science, Shiga University of Medical Science, Shiga, Japan
- Medical-risk Avoidance based on iPS Cells Team, RIKEN Center for Advanced Intelligence Projects (AIP), Kyoto, Japan
- AMED-CREST, Japan Agency for Medical Research and Development (AMED), Tokyo, Japan






