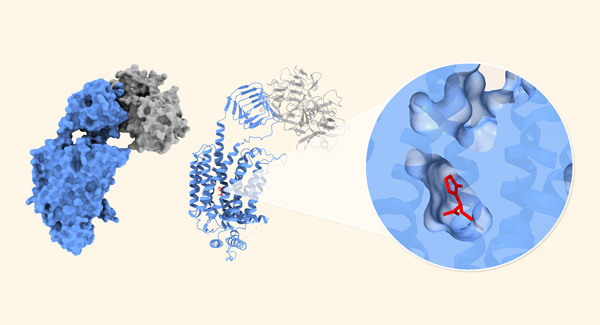
Figure 1: Left: Synaptic vesicle glycoprotein 2A (SV2A; blue) targeted by the botulinum neurotoxin receptor-binding domain (gray). Right: The antiepileptic drug levetiracetam (red) is bound inside SV2A. © RIKEN
RIKEN researchers have discovered how the structure of drugs for treating epilepsy allows them to interact with a key protein found in synapses at the junctions of neurons1. This knowledge could help to design even better drugs for the condition.
Epilepsy is a brain disorder that causes recurrent seizures, which can strike without warning. It is thought to affect somewhere between 0.5% and 1% of people.
Fortunately, powerful drugs are available for treating the neurological disorder, including levetiracetam and brivaracetam. But no-one knows exactly how these drugs work.
Both drugs target a protein found in the small, membrane-bound sacs called synaptic vesicles that store and release neurotransmitters at the ends of neurons. This protein is known as synaptic vesicle glycoprotein 2A (SV2A).
"The exact function of SV2A is unknown, although it must play a key role in synaptic transmission," says Atsushi Yamagata of the RIKEN Center for Biosystems Dynamics Research.
Interestingly, SV2A is also a receptor for the highly toxic botulinum neurotoxin. One of the most lethal substances known, botulinum neurotoxin can kill adults at levels of just a few hundred nanograms. But it is also used for a wide range of cosmetic and therapeutic purposes, including treating neuromuscular disorders, chronic pain and gastrointestinal disorders.
Now, by using cryo-electron microscopy, Yamagata and his co-workers have determined the structures of levetiracetam and brivaracetam when they are attached to SV2A, both when botulinum neurotoxin is present and absent (Fig. 1).
While the exact function of SV2A still remains unclear, the results suggest that SV2A may act as a membrane transporter and that levetiracetam and brivaracetam may inhibit this function.
The understanding of the structure "provides indirect evidence that SV2A functions as a membrane transporter," notes Yamagata. "This function might be very important for understanding epilepsy caused by genetic mutations of SV2A."
It is challenging to obtain a good-quality structure of SV2A using cryo-electron microscopy because the protein is quite small, but botulinum neurotoxin provided an unexpected benefit in this regard. "Luckily for us, when we attached a botulinum neurotoxin receptor-binding domain to SV2A, we could use it as a marker for cryo-electron microscopy image analysis," explains Yamagata. "That allowed us to align SV2A particle images more precisely and determine the high-resolution cryo-electron microscopy structure."
Yamagata and his team plan to extend this work. "We're now trying to determine the structure of SV2A in complex with full-length botulinum toxin. This may help us to understand how the neurotoxin functions in synapses," says Yamagata. "We also hope to elucidate the exact function of SV2A, although that's very challenging."






