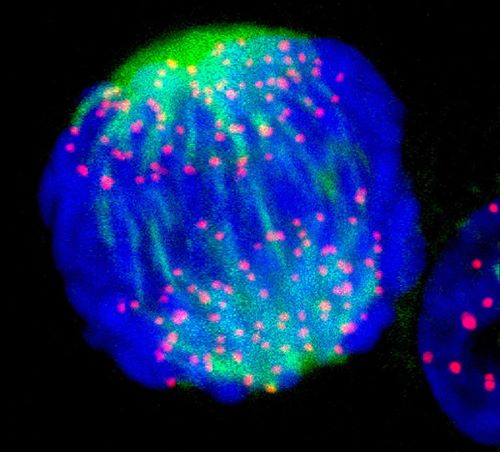
Figure 1: A fluorescence light micrograph of breast cancer cell about to divide into two. Chromosomes are stained blue, while the pink dots are kinetochores. RIKEN researchers have demonstrated artificial kinetochores that perform the same function using only two proteins. © DR MATTHEW DANIELS/SCIENCE PHOTO LIBRARY
In a demonstration that could help scientists better understand and manipulate cell division, RIKEN biologists have engineered artificial structures that replicate one of life's most crucial processes-the precise division of packages of DNA known as chromosomes1.
When a cell starts splitting into two daughter cells, its chromosomes align. The process of chromosome alignment can be likened to a high-stakes game of tug-of-war.
In a healthy cell, chromosomes line up at the center, each pulled by fibers extending from opposite sides of the cell. These fibers attach to kinetochores-anchors that ensure chromosomes are evenly pulled apart during cell division-at the center of the dividing structures (Fig. 1).
Balanced tension ensures that the chromosomes split evenly, with each new daughter cell receiving the correct genetic instructions. But when this balance falters, mistakes in chromosome distribution can happen, potentially leading to cancerous cells or genetic abnormalities.
Kinetochores are elaborate machines with dozens of proteins organized into layers-some interacting with the fibers that pull apart during cell division, others linking to the chromosomal DNA. But many of these proteins may be redundant for basic attachment and alignment.
Now, a team led by Tomoya Kitajima of the RIKEN Center for Biosystems Dynamics Research has shown that kinetochores can be artificially reduced to just two key proteins without losing their essential functionality in chromosome positioning.
Tethered onto tiny magnetic beads, these two proteins effectively created synthetic, miniaturized versions of kinetochores capable of anchoring fibers.
When injected into mouse cells, these artificial constructs performed just like their natural counterparts, joining the cellular tug-of-war and maintaining the critical tension needed to ensure accurate chromosome alignment.
This demonstration could lead to future innovations in synthetic biology and disease prevention.
"Artificial kinetochores could help us create new ways to artificially segregate DNA or other materials during cell division," says Kitajima. "This capability may be useful for building or controlling cells with custom functions."
Kitajima believes his designer kinetochores could serve as precision tools to synthetically guide biological processes that rely on the balanced tension between chromosomes. This has exciting potential for future medical applications.
In particular, they could open new doors for preventing or treating diseases caused by chromosome instability, a factor in genetic disorders such as cancer and Down syndrome.
In a related study, the researchers showed that their artificial kinetochore beads could help reduce chromosome-sorting errors in the egg cells of aging mice2.
"The effect of this error reduction was limited," says Kitajima. "But further improving the design of artificial kinetochores may allow us to prevent these chromosome errors more effectively."






