Metallic materials are the backbone of modern economies. However, large quantities of CO2 are produced during their production and processing. The metal industry must therefore use more climate-friendly processes in the future. The CO2 balance of alloys and their components must also be improved over their entire service life. Dierk Raabe, Director at the Max-Planck-Institut für Eisenforschung in Düsseldorf, explains the possibilities that industrial companies already have in this respect as well as the tasks that metallurgists must take on in order to achieve the goal of a sustainable metal industry.
Professor Raabe, what could the steel industry and other metalworking sectors do today to reduce their consumption of resources and their CO2 footprint quickly and noticeably?

Corrosion protection has a considerable effect because it makes products more durable. This is not only about iron, which rusts, but also other materials such as aluminium or nickel. It is also about corrosion by hydrogen, for example, which has a much more extreme effect on metals than water and oxygen. It can cause hydrogen embrittlement, damage that can lead to the sudden catastrophic failure of components. This was one of the causes of the Deep Water Horizon disaster, for example. However, it also plays a role in power plants, industrial buildings, and transport, especially if we want to rely more on hydrogen as a source of energy in the future. Even if corrosion protection doesn't sound so exciting to laypeople, it has considerable leverage because up to 4% of the world's economic output is destroyed by corrosion every year.
In which areas is corrosion a particularly big problem?
In some areas corrosion protection is already quite widespread. For example, in the automotive industry. There used to be an important question when buying a car: how quickly does it rust? That's now a thing of the past. However, industrial infrastructures, skyscrapers, bridges, power stations or trains - just think of the railway accident near Eschede in 1998 - are still highly susceptible to corrosion. And this will only multiply when hydrogen is added as an energy source in the next ten years.
Where do you see other opportunities to make steel and other metallic materials more sustainable?
The electrification of metal production will also have a major influence. Aluminium, the second most important metallic material after steel for the aircraft and automotive industries, has long been synthesized through the electrolytic reduction of aluminium ore. This requires a great deal of electricity, some of which is already obtained from renewable sources such as hydropower. You can also produce other metals - even iron - by electrolysis. However, this is not worthwhile because of the high electricity prices. All in all, electrification is one of the biggest levers for the sustainability of primary production and further processing of metals if the electricity comes exclusively from renewable sources.
What conditions are necessary to produce iron with electricity?
The sluggish expansion of the power lines for green electricity should finally speed up the pace. Because it must be clearly stated that in regions such as the Ruhr, where iron is produced, you will have to wait many more years for a connection to a green power supply sufficient for such industries as a glance at the homepage of the Federal Network Agency shows. In addition, market estimates by the Wuppertal Institute, for example, show that it could take up to 20 years before all-electric processes become competitive.
For the steel industry, however, this would mean that it would have to move from blast furnace production to completely new processes. Is that realistic?
Even for individual parts of integrated steelworks and aluminium smelters, the investment costs are so high that the industry cannot afford to rebuild them every ten years. Initially, however, the blast furnaces could even be left as they are. The industry can replace the carbon for reduction (i.e. coke, coal, biomass, and plastic waste) with up to 20% hydrogen, which would, of course, have to be generated from water using regenerative electricity. And because the steel industry accounts for around 6% of the world's total CO2 emissions, this would have a considerable impact. These processes are already being tested at several places around the world. The industry can also switch production to direct reduction in the medium term. The process involves filling granular oxide pellets (such as those supplied by mines after ore processing) as solids into a furnace and converting them directly with methane. This has long been done in countries where methane is affordable. This process has the advantage that the plants can, in principle, be converted to up to 100% hydrogen.
So when will iron be smelted with hydrogen?
The completely hydrogen-based process will need 10 to 12 years before it can be placed on the market. It is estimated that they will be approx. 30% more expensive than current blast furnace production. And the CO2 price increase has not yet been fully determined. It may therefore be that in 10 years, a 30% increased will be a competitive market price if correspondingly less sustainable competing materials from outside the EU are subjected to comparable conditions. The worst of all solutions would be for metal production to disappear from Europe and for us to buy unsustainable metals from countries outside the EU. Europe needs an independent and sustainable metal producing and processing industry, not least because it generates around €400 billion per year.
What interest could industry in countries like Germany have in exchanging their plants for direct reduction plants?

On one hand, the steel industry can produce iron in a CO2-reduced manner. Companies already see the necessity for this because they can estimate that the costs will rise in the coming years because of CO2 pricing and because car manufacturers, for example, hope to utilize an increasing fraction of CO2-reduced steel in the future. On the other hand, the direct reduction also enables companies to become more flexible. A blast furnace must be kept running continuously. Otherwise, it will break down. With furnaces for direct reduction, companies can adapt much more flexibly to the market and produce steels in various qualities. We are also surprised that the steel industry is already planning and setting about the conversion to such plants on a massive scale worldwide. Some existing plants are already being converted to hydrogen. In the new few years, the metal industry will undergo one of the greatest upheavals in history. For over 3500 years, iron has (in principle) been produced using the same reduction process.
What political framework conditions must be created to make metal production more sustainable?
When making political decisions, we should, in any case, analyse how legislative measures such as subsidies or bans affect the CO2 balance over complete life cycles. For example, if you pumped a lot of money into producing steel completely electrolytically, it would sound great. However, a look at the electricity mix shows that, as with the electric car, there is still 25% brown coal electricity. Then we haven't gained anything. Sustainability must also be thought through in a sustainable way. It's no use showing off.
In your opinion, where would legal regulations make sense?
For example, in incentives for closed scrap cycles in industry. I'll give you an example: There are some automobile companies that already mainly produce only aluminium cars in the premium segment and, in some cases, process up to 300,000 tonnes of aluminium annually. However, when the components are punched out of the sheet metal, up to 45% of the material is lost. Now you'd think they'd collect their own scrap. Because when the aluminium is so pure, it's like cash in hand. But only a few companies do this consistently. For example, here in the EU. Otherwise it is still much cheaper for many companies to buy new material on the market instead of establishing closed scrap cycles. And most scrap metal is also already mixed, which reduces its value to as low as one tenth. For example, creating tax incentives for separate scrap cycles at an early stage would do much more than simply collecting coffee capsules or foil wrappers, which we as consumers produce. That isn't to say we shouldn't be concerned with them. But compared with industrial waste, it is a matter of decimal places.
What research needs do you see for sustainable metal materials?
At the moment, many different alloys are used in many products because they all have some special property. Initially, we look at which elements occur in alloys when a certain amount of scrap is used. For example, you can already find the extremely expensive neodymium from the electric motors of window winders and the like in the recycled aluminium used in cars today, because they are not separated before they are melted down. We thus find over 20 elements in alloys that we hadn't had before. We are investigating how such impurities change the properties of alloys. We hope to find out how impure a material can be and still fulfil its purpose. If we can scientifically prove that a material can be less pure, we can increase the scrap content and thus massively reduce the CO2 footprint.
Can scrap from one industry be recycled in another?
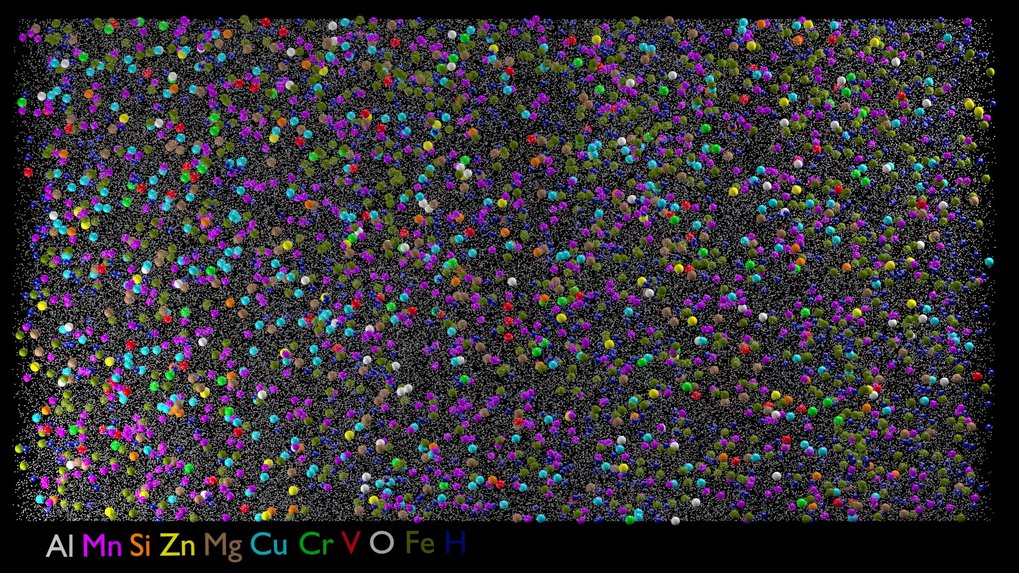
We are looking into such possibilities. We systematically look at where a lot of material is consumed and whether we can make alloys that can tolerate more impurities. For example, we have found that the construction industry is using increasingly more aluminium alloys related to the aluminium-manganese alloy of beverage cans for roof tiles, cladding, load-bearing elements, lifts, and the like. In the case of cans, the proportion of recycling and thus the amount of impurities is already quite high, because the alloy is relatively good-natured and does not have to be able to do much. We now want to investigate whether the can scrap, which many countries produce in larger quantities than in Germany, can also be used for construction purposes.
What is the second step for research?
We are trying to reduce the number of alloys and develop a kind of unitary alloy. This would be much better to recycle because much less sorting would be required. Until now, the specialization of materials has always been obtained at the price of a chemical change: Material scientists fiddle with the chemical composition until the fender, aircraft component, or turbine gets better. We would like to reduce this extreme diversification of varieties, which makes recycling difficult. A specific example: a car manufacturer could demand that a steel or aluminium producer use only two alloys instead of five, all of which have been perfected to impart a certain property such as strength or surface quality.
How could the diversity of alloys be limited?
The fundamental question here is whether we can achieve diversification not only through chemical composition but also primarily through changes in the micro- and nano-structure. This has traditionally worked well with metals. However, you must invest a lot more effort in the production in order to achieve a certain size and orientation of the crystals (as an example). This approach shifts the basic approach of material production from materials chemistry to metal physics.
How many alloys do you expect would remain?
For example, if you purchase an aluminium alloy today, you can choose between up to 280 alloys that can do anything that aluminium should be able to do. But if you look at what is really sold in large quantities, there are only 50 or 60 alloys left. And if you take a closer look at exactly what these alloys are supposed to achieve, you might end up with only 20 or 30 alloys. Of course, that's just a rough estimate.
The CO2 emissions of the metal industry could also be reduced by using less material. Do you see possibilities to make car bodies lighter, for example?
First of all: cars have become bigger and heavier in the past decades, partly because of additional equipment such as air conditioning, wiring, or on-board computers, which are considered the minimum standard today. And of course the situation is quite extreme with electric vehicles in which the battery alone weighs up to 800 kg. But you could add another 200 or 300 kg if the bodies hadn't already become much lighter because the alloys were getting harder and harder. Nevertheless, the competition among material manufacturers is still continuing to see who can supply the strongest steels and aluminium alloys. Because we are still at only about one tenth of the theoretically possible strength of these materials. So there is still a lot of research to be done to bring the materials to their physical limits.
Perhaps you as a metal researcher are not the right addressee for the next question. Nevertheless: would it make sense to replace metallic materials with plastics in some places?
(Laughs) You really are asking the wrong person. In fact, polymer materials with carbon fibres have been propagated time and again for car bodies. But in terms of the ecological balance, this is really nonsense. The production of carbon fibres requires an extremely high amount of energy and releases large amounts of CO2. And in the end, you can only throw most of these materials into the waste incineration plant. It is often stated that these polymer-based materials can be recycled. But you can really only chop them up and make mats out of them. Metals, on the other hand, can be recycled infinitely often, provided that the scrap is collected by type, the effect of impurities is understood and controlled, and the variety of alloys used is reduced. And lightweight magnesium components already come very close to polymer components in terms of weight but are completely recyclable.
Interview: Peter Hergersberg






