Everyone knows that burning coal causes air pollution that is harmful to the climate and human health. But the ash left over can often be harmful as well.
For example, Duke Energy long stored a liquified form of coal ash in 36 large ponds across the Carolinas. That all changed in 2014, when a spill at its Dan River site released 27 million gallons of ash pond water into the local environment. The incident raised concerns about the dangers associated with even trace amounts of toxic elements like arsenic and selenium in the ash. Little was known, however, about just how much of these hazardous materials were present in the ash water or how easily they could contaminate the surrounding environment.
Fears of future spills and seepage caused Duke Energy to agree to pay $1.1 billion to decommission most of its coal ash ponds over the coming years. Meanwhile, researchers are working on better ways of putting the ash to use, such as recycling it to recover valuable rare earth elements or incorporating it into building materials such as concrete. But to put any potential solution into action, researchers still must know which sources of coal ash pose a hazardous risk due to its chemical makeup - a question that scientists still struggle to answer.
"We saw arsenic and selenium either attached to the surface of fine grain particles or encapsulated within them, which explains why these elements leach out of some coal ash sources more readily than others."
helen hsu-kim
In a new paper published June 6 in the journal Environmental Science: Nano, researchers at Duke University have discovered that these answers may remain elusive because nobody is thinking small enough. Using one of the newest, most advanced synchrotron light sources in the world - the National Synchrotron Light Source II at Brookhaven National Laboratory - the authors show that, at least for selenium and arsenic, the amount of toxic elements able to escape from coal ash depends largely on their nanoscale structures.
"These results show just how complex coal ash is as a material," said Helen Hsu-Kim, professor of civil and environmental engineering at Duke University. "For example, we saw arsenic and selenium either attached to the surface of fine grain particles or encapsulated within them, which explains why these elements leach out of some coal ash sources more readily than others."
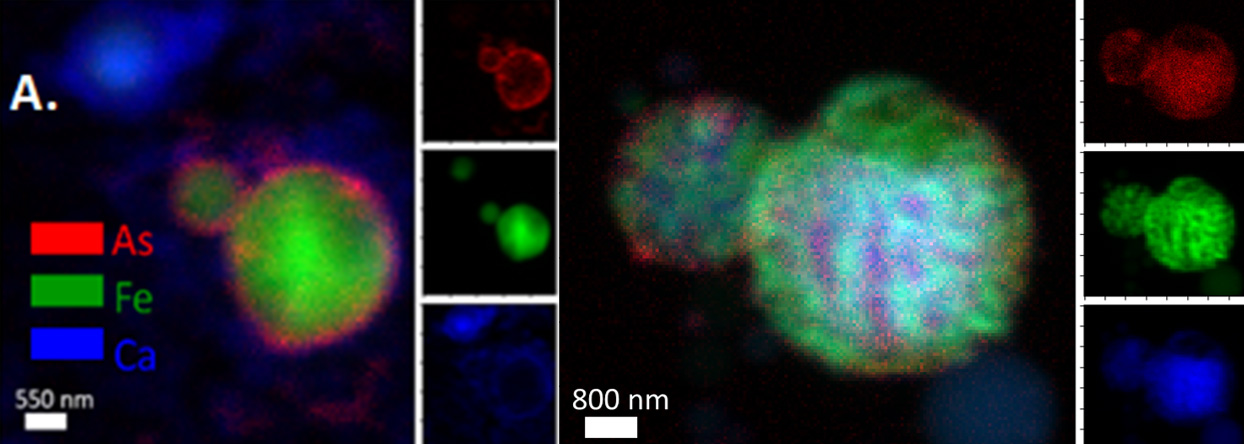
It's long been known that factors in the surrounding environment such as pH affect how well toxic elements can move from source to surroundings. In previous research, Hsu-Kim showed that the amount of oxygen in a toxin's surroundings can greatly affect its chemistry, and that different sources of coal ash produce vastly different levels of byproducts.
But just because one source of coal ash is high in arsenic doesn't necessarily mean that high amounts of arsenic will leach out of it. Similarly, various sources of ash respond differently to the same environmental conditions. The problem is complex, to say the least. To take a different approach, Hsu-Kim decided to take an even closer look at the source itself.
"Researchers in the field typically use x-ray microscopy with a resolution of one or two micrometers, which is about the same size as the fly ash particles themselves," Hsu-Kim said. "So if a single particle is a single pixel, you're not seeing how the elements are distributed across it."
"Researchers in the field typically use x-ray microscopy with a resolution of one or two micrometers, which is about the same size as the fly ash particles themselves. So if a single particle is a single pixel, you're not seeing how the elements are distributed across it."
helen hsu-kim
To shrink these pictures' pixels to the nanoscale, Hsu-Kim turned to Catherine Peters, professor of civil and environmental engineering at Princeton University, and her colleagues to acquire time on the National Synchrotron Light Source II. The futuristic machine creates light beams 10 billion times brighter than the sun to reveal the chemical and atomic structure of materials using light beams ranging from infrared to hard X-rays.
Brookhaven's capabilities were able to provide the researchers a nanoscale map of each particle along with the distribution of elements in each particle. The incredible resolution revealed that coal ash is a compilation of particles of all kinds and sizes.
 For example, in one sample the researchers saw individual nanoparticles of selenium that were attached to bigger particles of coal ash, which is a chemical form of selenium that probably isn't very soluble in water. But most of the ash had arsenic and selenium either locked inside individual grains or attached at the surface with relatively weak ionic bonds that are easily broken.
For example, in one sample the researchers saw individual nanoparticles of selenium that were attached to bigger particles of coal ash, which is a chemical form of selenium that probably isn't very soluble in water. But most of the ash had arsenic and selenium either locked inside individual grains or attached at the surface with relatively weak ionic bonds that are easily broken.
"It was almost like we saw something different in every sample we looked at," Hsu-Kim said. "The wide array of differences really highlights why the main characteristic that we care about - how much of these elements leach out of the ash - varies so much between different samples."
While nobody can say for sure what causes the coal ash to develop its unique composition, Hsu-Kim guesses that it is likely mostly related to how the coal was originally formed millions of years ago. But it might also have something to do with the power plants that burn the coal. Some plants inject activated carbon or lime into the flue gas, which captures mercury and sulfur emissions, respectively. At 1000 degrees Fahrenheit, toxins such as arsenic and selenium in the flue are gaseous, and the physics that dictate how the particles will cool and recombine to form ash is uncontrollable.
But regardless of the how, researchers now know that they should be paying closer attention to the fine details encapsulated within the end results.
This work was supported by the U.S. Department of Energy (DE-FE0031748) and the National Institute of Environmental Health Sciences (5U2C-ES030851). This research utilized U.S. DOE Office of Science User Facility resources at the Stanford Synchrotron Radiation Lightsource facility operated by SLAC National Accelerator Laboratory (DE-AC02-76SF0051) and at the Hard X-ray Nanoprobe (HXN) Beamline at 3-ID of the National Synchrotron Light Source II facility operated by Brookhaven National Laboratory (DE-SC0012704).
CITATION: "Nanoscale Heterogeneity of Arsenic and Selenium Species in Coal Fly Ash Particles: Analysis using enhanced spectroscopic imaging and speciation techniques," Nelson A. Rivera, Jr, Florence T. Ling, Ajith Pattammattel, Hanfei Yan, Yong S. Chu, Catherine Peters, Heileen Hsu-Kim. Environmental Science: Nano, June 6, 2023. DOI: 10.1039/D2EN01056A






