A research team led by Prof. LUAN Fubo from the Research Center for Eco-Environmental Sciences of the Chinese Academy of Sciences, has uncovered a novel mechanism involving a ternary surface complex on titanium dioxide (TiO₂) that improves the simultaneous removal of arsenic (As) and uranium (U) from contaminated groundwater. This study was published in Proceedings of the National Academy of Sciences (PNAS).
Groundwater contamination by arsenic and uranium is a severe environmental and public health concern, linked to cancer, kidney damage, and other chronic diseases. Conventional remediation methods typically target either arsenic or uranium, often proving ineffective when both contaminants coexist due to competitive adsorption effects.
In this study, the researchers demonstrated that TiO₂ nanoparticles can simultaneously and efficiently adsorb both As(V) and U(VI). Notably, uranium enhanced arsenic adsorption, increasing removal efficiency by up to 3.4 times compared to arsenic-only systems.
Using in situ ATR-FTIR spectroscopy and density functional theory (DFT) calculations, the researchers identified the formation of a ternary surface complex, [Ti-U(VI)-As(V)], as the critical mechanism. In this process, arsenate ions displace carbonate ligands in uranyl-carbonate complexes adsorbed on TiO₂, forming a stable ternary structure that facilitates co-removal of both pollutants.
The adsorption process proved effective under typical groundwater conditions, achieving >99% removal efficiency for both contaminants. Post-treatment residual concentrations fell below the safety limits of the World Health Organization for drinking water.
An advantage of this method is the regenerability and reusability of the TiO₂ adsorbent. The captured arsenic and uranium can be recovered using a mild sodium hydroxide solution, allowing the material to be reused multiple times without significant loss of performance.
This study provides a cost-effective, scalable, and eco-friendly approach to remediating dual-contaminated groundwater, offering the potential for improving global drinking water safety.
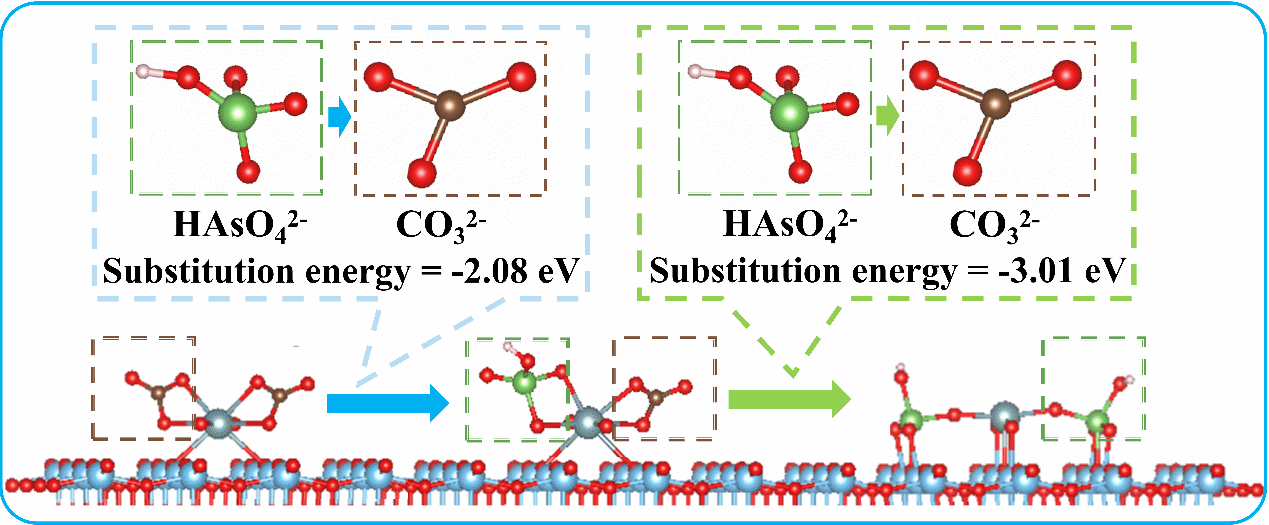
Formation Mechanism of the Ternary Complex [Ti-U(VI)-As(V)] (Image by LI Zheng)






