Researchers from Iwate Medical University and Tohoku University have revealed that it is possible to predict cancer relapse and treatment response by measuring circulating tumor DNA (ctDNA), in tandem with data from comprehensive genomic profiling (CGP).
CGP test results can be used to tailor recommendations for advanced cancer treatment, with the goal of matching individual patients with the most effective option to prolong survival. This study retrospectively looked at the CGP and clinical data of 219 patients of Iwate Medical University with advanced cancer registered with the Center for Personalized Medicine at Tohoku University Hospital. Of these patients, only 14 (6.4%) received a tailored treatment based on their CGP results.
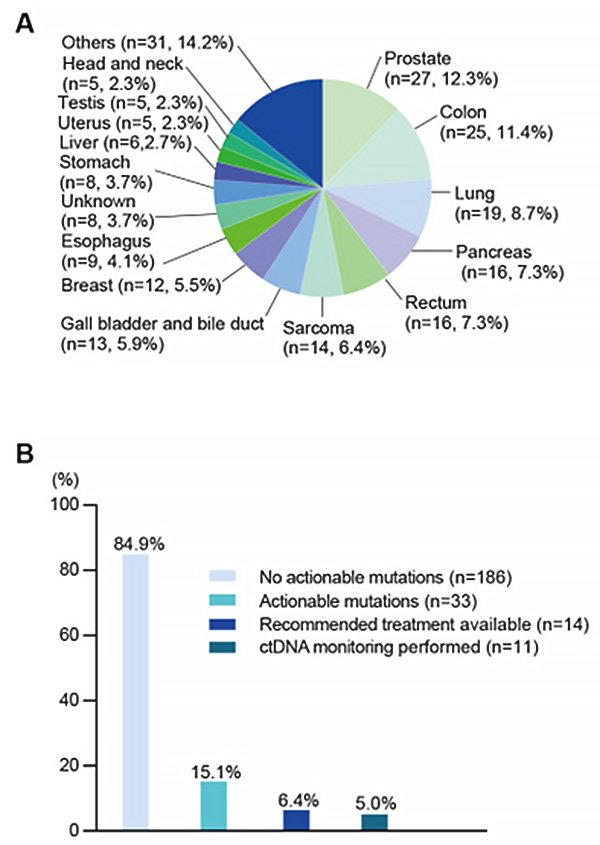
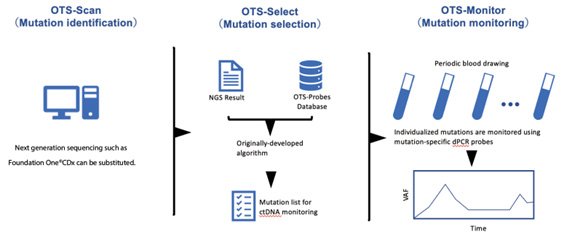
The research team proposed that patients who underwent CGP could undergo ctDNA monitoring by using digital PCR (dPCR) (Off The Shelf-assay, hereafter OTS-Assay). The OTS-Assay was originally developed at Iwate Medical University, and has been clinically validated for a number of different cancers. Strengthening our knowledge and further testing the validity of this system is crucial, so that it can potentially become more commonplace in clinical practice.
"First, we complete CGP to recommend an ideal treatment. Then, we can further predict and monitor the efficacy of that treatment using a relevant ctDNA - a measure of tumor DNA in the blood that can pick up even minimal hints that the cancer is still persisting," explains Satoshi Nishizuka (Iwate Medical University).
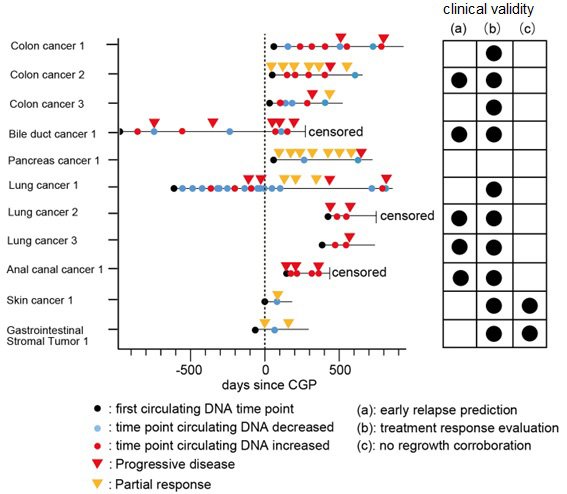
The clinical validity of the OTS-Assay was evaluated for "early relapse prediction," "treatment response evaluation," and "no relapse/regrowth corroboration". Monitoring of ctDNA by dPCR was performed in 11 patients. Overall, 10 of 11 patients (90.9%) achieved at least one of the following validated outcomes: "early relapse prediction," "treatment response evaluation," and "no relapse/regrowth corroboration".
This study demonstrated that ctDNA monitoring using the OTS-Assay system can be used effectively in conjunction with CGP data in order to predict patient outcomes for a broad range of cancer types. This finding represents a step forward for personalized precision medicine. Importantly, the majority of patients who receive CGP but do not actually receive a recommended treatment (the remaining 93.6%) can still undertake ctDNA monitoring using the OTS-Assay system to evaluate their disease progression. This type of personalized medical care could improve clinical management techniques, and ultimately help cancer patients around the world.
- Publication Details:
Title: Comprehensive genome profiling-initiated tumor-informed circulating tumor DNA monitoring for patients with advanced cancer
Authors: Taiga Sasaki, Hayato Hiraki, Akiko Yashima-Abo, Hiromi Nagashima, Fumitaka Endo, Mizunori Yaegashi, Shimpei Miura, Keiko Obata, Naoki Yanagawa, Hiroaki Itamochi, Hidekazu Shirota, Takeshi Iwaya, Satoshi S. Nishizuka
Journal: Cancer Science
DOI: 10.1111/cas.16446






