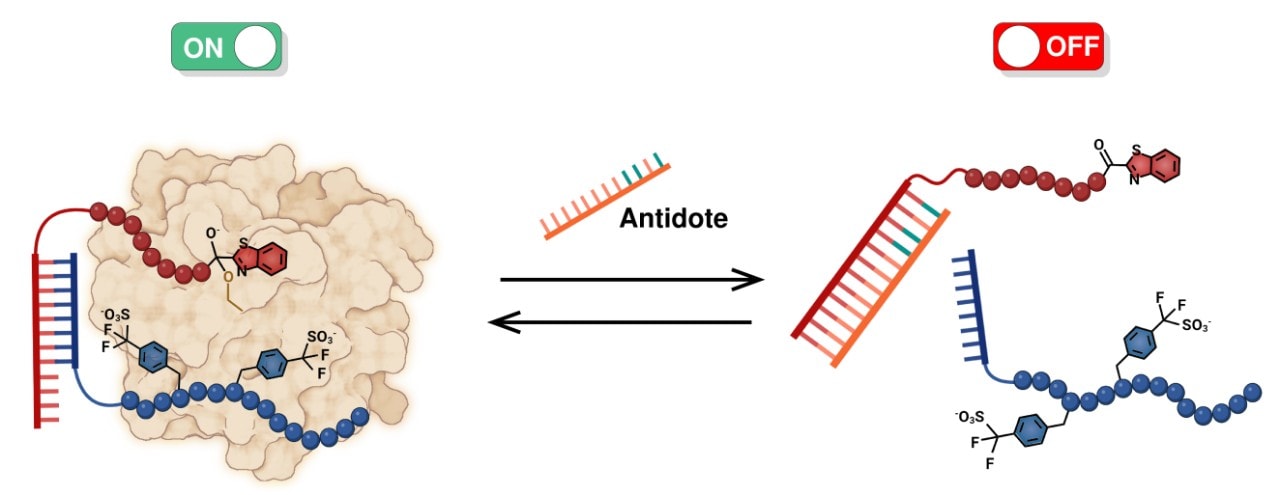
The image illustrates the combined action of two peptide molecules cooperating to inhibit thrombin. The antidote PNA (peptide nucleic acid) dissociates the two molecules, which 'switches off' the anti-clotting action of the combined molecule.
The blue molecule is the peptide fragment derived from the tsetse fly, the red is the active site binder with the ketobenzothiazole fragment. They are 'joined' by PNA, non-convalent bonding. Source: University of Geneva
Researchers at the University of Sydney and University of Geneva have developed a new anticoagulant, whose anticlotting action can be rapidly stopped 'on demand'. The result could lead to new surgical and post-operative drugs that minimise the risk of serious bleeding.
The research team applied a completely new method to discover the molecule. The anticoagulant combines a short protein molecule (a peptide) from a tsetse fly - a blood-feeding insect - with a second, synthesised peptide. The bonds holding the two peptides together can be broken on demand, providing the anticoagulant ingredient with its own on-off switch.
This new drug-discovery approach is a potential game-changer in surgery and for suppressing blood clots. It could also be applied in other fields such as immunotherapy.
The results are published today in Nature Biotechnology.
In addition to surgical applications, anticoagulant therapies are essential for managing a wide range of conditions, such as heart disease, stroke and venous thrombosis. However, current treatment options, such as heparin and warfarin, have major drawbacks, including the need for regular monitoring of blood coagulation and the risk of serious bleeding in the event of overdose.
About 15 percent of emergency hospital admissions due to adverse drug reactions are attributable to complications from anticoagulant treatments, emphasising the importance of developing new, safer and more effective therapeutic options.
Professor Rich Payne from the School of Chemistry is an NHMRC Investigator Leadership Fellow and Deputy Director of the ARC Centre of Excellence for Innovations in Peptide and Protein Science (CIPPS) and is a coauthor of the research.

Professor Rich Payne from the School of Chemistry and CIPPS.
He said: "What's exciting here is that we have applied a completely novel approach to drug discovery. The anticoagulant we have developed uses what we call supramolecular chemistry. This allows the two active molecules needed to suppress coagulation to self-assemble.
"The architecture also means we can apply an antidote that can quickly disassemble the joined molecules, triggering a rapid cessation of the active combination and the anticoagulant effect.
"This has never been done before in drug discovery."
Research lead, Professor Nicolas Winssinger from the Department of Organic Chemistry at the University of Geneva, said: "This result goes beyond the development of a new anticoagulant and its associated antidote. The supramolecular approach proposed is remarkably flexible and can be easily adapted to other therapeutic targets. It is particularly promising in the field of immunotherapy."
Revolution for surgery
The new anticoagulant could offer a more reliable and easier-to-use option for surgical procedures. Heparin, commonly used in this field, is a mixture of polymers of different lengths extracted from pig intestine. The use of heparin in the clinic is problematic due to the risk of serious bleeding side effects and requires coagulation tests during surgery. The new synthetic anticoagulant developed by the Geneva and Sydney team could help solve the problems of purity and availability associated with heparin.

Professor Nicolas Winssinger. University of Geneva.
One of the breakthroughs in this work lies in the use of a peptide nucleic acid (PNA) to link the two molecules that bind and block the action of thrombin, the enzyme that produces fibrin that makes up our blood clots.
In this case, the tsetse-fly-derived peptide molecule and a synthetic ketobenzothiazole containing peptide bind to two distinct sites on thrombin as a 'supramolecule' connected by a PNA double helical linker, similar in shape to DNA.
These two strands of PNA that make up the double helix can come together via relatively weak - non-covalent - bonds that can be broken when needed. The research team has shown that by introducing correctly matched strands of free PNA, it is possible to dissociate the two thrombin-binding molecules. The two free PNA strands are no longer active as anticoagulants. This is a major innovation in the field.
The tsetse fly peptide was developed in laboratories at the University of Sydney. Tests for the efficacy of the supramolecular anticoagulant were also tested at Sydney in human and mouse blood samples and also in vivo in mice.
Useful for immunotherapy
Beyond the problem of anticoagulation, this supramolecular concept of activating and deactivating the active principle could be of significant interest in the field of immunotherapy, particularly for CAR-T therapies.
Although CAR-T therapies are one of the major advances in the treatment of certain cancers in recent years, their use is associated with a significant risk of immune system storm, which can be fatal. The ability to rapidly deactivate treatment with an accessible antidote could be a crucial advance in improving the safety and efficacy of CART-T therapies.
Research
Dockerill, M. et al. 'Development of supramolecular anticoagulants with on-demand reversibility'. Nature Biotechnology. DOI: 10.1038/s41587-024-02209-z
Declaration
The research partnership was made possible thanks to a seed grant of $10,000 from the University of Sydney Development Office.
The research was supported in part by the National Health and Medical Research Council (Australia), Swiss National Science Foundation, NCCR Chemical Biology, Fundação para a Ciêcia e a Tecnologia (Portugal), the Australian Research Council and the European Union.
The authors declare no competing interest.






